Critical roles of sphingosine kinase 1 in the regulation of neuroinflammation and neuronal injury after spinal cord injury
- PMID: 33602274
- PMCID: PMC7893778
- DOI: 10.1186/s12974-021-02092-4
Critical roles of sphingosine kinase 1 in the regulation of neuroinflammation and neuronal injury after spinal cord injury
Abstract
Background: The pathological process of traumatic spinal cord injury (SCI) involves excessive activation of microglia leading to the overproduction of proinflammatory cytokines and causing neuronal injury. Sphingosine kinase 1 (Sphk1), a key enzyme responsible for phosphorylating sphingosine into sphingosine-1-phosphate (S1P), plays an important role in mediating inflammation, cell proliferation, survival, and immunity.
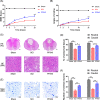
Methods: We aim to investigate the mechanism and pathway of the Sphk1-mediated neuroinflammatory response in a rodent model of SCI. Sixty Sprague-Dawley rats were randomly assigned to sham surgery, SCI, or PF543 (a specific Sphk1 inhibitor) groups. Functional outcomes included blinded hindlimb locomotor rating and inclined plane test.
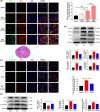
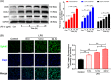
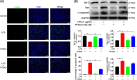
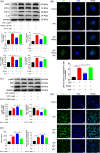
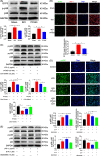
Results: We discovered that Sphk1 is upregulated in injured spinal cord tissue of rats after SCI and is associated with production of S1P and subsequent NF-κB p65 activation. PF543 attenuated p65 activation, reduced inflammatory response, and relieved neuronal damage, leading to improved functional recovery. Western blot analysis confirmed that expression of S1P receptor 3 (S1PR3) and phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) are activated in microglia of SCI rats and mitigated by PF543. In vitro, we demonstrated that Bay11-7085 suppressed NF-κB p65 and inhibited amplification of the inflammation cascade by S1P, reducing the release of proinflammatory TNF-α. We further confirmed that phosphorylation of p38 MAPK and activation of NF-κB p65 is inhibited by PF543 and CAY10444. p38 MAPK phosphorylation and NF-κB p65 activation were enhanced by exogenous S1P and inhibited by the specific inhibitor SB204580, ultimately indicating that the S1P/S1PR3/p38 MAPK pathway contributes to the NF-κB p65 inflammatory response.
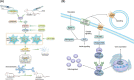
Conclusion: Our results demonstrate a critical role of Sphk1 in the post-traumatic SCI inflammatory cascade and present the Sphk1/S1P/S1PR3 axis as a potential target for therapeutic intervention to control neuroinflammation, relieve neuronal damage, and improve functional outcomes in SCI.
Keywords: M1 microglia; NF-κB p65 activation; S1P/S1PR3/p38 MAPK pathway; Sphk1; Spinal cord injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

