Clinicopathologic and Genomic Analysis of TP53-Mutated Endometrial Carcinomas
- PMID: 33602681
- PMCID: PMC8530276
- DOI: 10.1158/1078-0432.CCR-20-4436
Clinicopathologic and Genomic Analysis of TP53-Mutated Endometrial Carcinomas
Abstract
Purpose: Copy number-high endometrial carcinomas were described by The Cancer Genome Atlas as high-grade endometrioid and serous cancers showing frequent copy-number alterations (CNA), low mutational burden (i.e., non-hypermutant), near-universal TP53 mutation, and unfavorable clinical outcomes. We sought to investigate and compare the clinicopathologic and molecular characteristics of non-hypermutant TP53-altered endometrial carcinomas of four histologic types.
Experimental design: TP53-mutated endometrial carcinomas, defined as TP53-mutant tumors lacking microsatellite instability or pathogenic POLE mutations, were identified (n = 238) in a cohort of 1,239 endometrial carcinomas subjected to clinical massively parallel sequencing of 410-468 cancer-related genes. Somatic mutations and CNAs (n = 238), and clinicopathologic features were determined (n = 185, initial treatment planning at our institution).
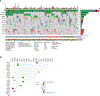
Results: TP53-mutated endometrial carcinomas encompassed uterine serous (n = 102, 55.1%), high-grade endometrial carcinoma with ambiguous features/not otherwise specified (EC-NOS; n = 44, 23.8%), endometrioid carcinomas of all tumor grades (n = 28, 15.1%), and clear cell carcinomas (n = 11, 5.9%). PTEN mutations were significantly more frequent in endometrioid carcinomas, SPOP mutations in clear cell carcinomas, and CCNE1 amplification in serous carcinomas/EC-NOS; however, none of these genomic alterations were exclusive to any given histologic type. ERBB2 amplification was present at similar frequencies across TP53-mutated histologic types (7.7%-18.6%). Although overall survival was similar across histologic types, serous carcinomas presented more frequently at stage IV, had more persistent and/or recurrent disease, and reduced disease-free survival.
Conclusions: TP53-mutated endometrial carcinomas display clinical and molecular similarities across histologic subtypes. Our data provide evidence to suggest performance of ERBB2 assessment in all TP53-mutated endometrial carcinomas. Given the distinct clinical features of serous carcinomas, histologic classification continues to be relevant.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 2018;29:1180–8. - PubMed
-
- Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol Oncol 2020;158:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

