Selenomethionine as an expressible handle for bioconjugations
- PMID: 33602807
- PMCID: PMC7923357
- DOI: 10.1073/pnas.2005164118
Selenomethionine as an expressible handle for bioconjugations
Abstract
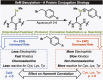
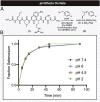
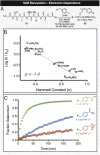
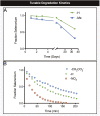
Site-selective chemical bioconjugation reactions are enabling tools for the chemical biologist. Guided by a careful study of the selenomethionine (SeM) benzylation, we have refined the reaction to meet the requirements of practical protein bioconjugation. SeM is readily introduced through auxotrophic expression and exhibits unique nucleophilic properties that allow it to be selectively modified even in the presence of cysteine. The resulting benzylselenonium adduct is stable at physiological pH, is selectively labile to glutathione, and embodies a broadly tunable cleavage profile. Specifically, a 4-bromomethylphenylacetyl (BrMePAA) linker has been applied for efficient conjugation of complex organic molecules to SeM-containing proteins. This expansion of the bioconjugation toolkit has broad potential in the development of chemically enhanced proteins.
Keywords: bioconjugation; protein chemistry; selenomethionine.
Conflict of interest statement
The authors declare no competing interest.
Figures
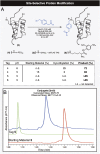
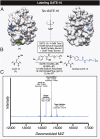
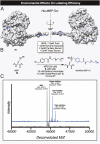
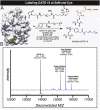
References
-
- Stephanopoulos N., Francis M. B., Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 7, 876–884 (2011). - PubMed
-
- Beck A., Goetsch L., Dumontet C., Corvaïa N., Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

