Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks
- PMID: 33602874
- PMCID: PMC7919413
- DOI: 10.1101/gad.346460.120
Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks
Abstract
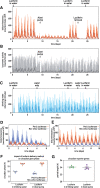
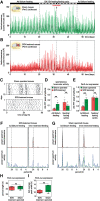
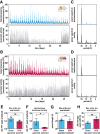
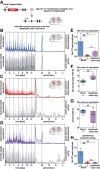
It has been assumed that the suprachiasmatic nucleus (SCN) synchronizes peripheral circadian oscillators. However, this has never been convincingly shown, since biochemical time series experiments are not feasible in behaviorally arrhythmic animals. By using long-term bioluminescence recording in freely moving mice, we show that the SCN is indeed required for maintaining synchrony between organs. Surprisingly, however, circadian oscillations persist in the livers of mice devoid of an SCN or oscillators in cells other than hepatocytes. Hence, similar to SCN neurons, hepatocytes can maintain phase coherence in the absence of Zeitgeber signals produced by other organs or environmental cycles.
Keywords: circadian gene expression; in vivo bioluminescence recording; liver; suprachiasmatic nucleus.
© 2021 Sinturel et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
