Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment
- PMID: 33602901
- PMCID: PMC7892878
- DOI: 10.1038/s41408-021-00430-6
Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment
Abstract
Epcoritamab (DuoBody-CD3xCD20, GEN3013) is a novel bispecific IgG1 antibody redirecting T-cells toward CD20+ tumor cells. Here, we assessed the preclinical efficacy of epcoritamab against primary tumor cells present in the lymph node biopsies from newly diagnosed (ND) and relapsed/refractory (RR) B-NHL patients. In the presence of T-cells from a healthy donor, epcoritamab demonstrated potent activity against primary tumor cells, irrespective of prior treatments, including CD20 mAbs. Median lysis of 65, 74, and 84% were achieved in diffuse large B-cell lymphoma (n = 16), follicular lymphoma (n = 15), and mantle cell lymphoma (n = 8), respectively. Furthermore, in this allogeneic setting, we discovered that the capacity of B-cell tumors to activate T-cells was heterogeneous and showed an inverse association with their surface expression levels of the immune checkpoint molecule Herpesvirus Entry Mediator (HVEM). In the autologous setting, when lymph node (LN)-residing T-cells were the only source of effector cells, the epcoritamab-dependent cytotoxicity strongly correlated with local effector cell-to-target cell ratios. Further analyses revealed that LN-residing-derived or peripheral blood-derived T-cells of B-NHL patients, as well as heathy donor T-cells equally mediated epcoritamab-dependent cytotoxicity. These results show the promise of epcoritamab for treatment of newly-diagnosed or relapsed/refractory B-NHL patients, including those who became refractory to previous CD20-directed therapies.
Conflict of interest statement
T.M., D.J. received research funding from Genmab. H.J.H., M.E.C., E.C.W.B., and T.M. are inventors on Genmab patent applications. I.H.H. and E.C.W.B. own Genmab warrants and/or stock.
Figures

 and
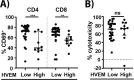
and  , respectively). Statistical analysis was performed with Kruskal–Wallis and Dunn’s multiple comparisons test to compare epcoritamab with CD3xCtrl for each indication (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001) and epcoritamab-dependent cytotoxicity between subtypes (ns (C) CD20 expression (antibody molecules per cell) on tumor cells in samples from B-NHL patients unexposed to prior CD20 therapy correlated to epcoritamab-dependent cytotoxicity (30 ng/mL) (Spearman’s; ns). D Time between last CD20-therapy and moment of lymph node biopsy (months) of patients previously treated with CD20-antibody containing treatment (n = 8) correlated to cytotoxicity mediated by epcoritamab (30 ng/mL) (Spearman’s; r = 0.78, *p = 0.03).
, respectively). Statistical analysis was performed with Kruskal–Wallis and Dunn’s multiple comparisons test to compare epcoritamab with CD3xCtrl for each indication (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001) and epcoritamab-dependent cytotoxicity between subtypes (ns (C) CD20 expression (antibody molecules per cell) on tumor cells in samples from B-NHL patients unexposed to prior CD20 therapy correlated to epcoritamab-dependent cytotoxicity (30 ng/mL) (Spearman’s; ns). D Time between last CD20-therapy and moment of lymph node biopsy (months) of patients previously treated with CD20-antibody containing treatment (n = 8) correlated to cytotoxicity mediated by epcoritamab (30 ng/mL) (Spearman’s; r = 0.78, *p = 0.03).

 and
and  , respectively). Statistical analysis was performed with Kruskal–Wallis and Dunn’s multiple comparisons test to compare epcoritamab with CD3xCtrl (*p < 0.05) and to compare epcoritamab-dependent cytotoxicity (subtracted with CD3xCTRL values) between subtypes (ns). B Ratio of CD4+ T helper cells (CD4+CD25−) to CD8+ T-cells in DLBCL, FL, and MCL samples (Kruskal–Wallis with Dunn’s multiple comparisons test; *p < 0.05, **p ≤ 0.01) (median ± interquartile range). C PD-1 expression (MFI values normalized to expression on T-cells of internal healthy donor control) on CD4+ T-cells of DLBCL, FL, including PD-1 bright and dim populations, and MCL (Kruskal–Wallis with Dunn’s multiple comparisons test; *p < 0.05, **p < 0.01, ***p < 0.001) (median ± interquartile range). D Spearman’s correlation between CD3:Target ratio and cytotoxicity mediated by epcoritamab (30 ng/mL) (r = 0.63; ****p < 0.0001). E Lymph node T-cells of B-NHL patients isolated by magnetic-activated cell-sorting (MACS) showed similar percentages B-cell lymphoma cell line (WSU-DLCL2 and Daudi, left to right) cytotoxicity as T-cells isolated from healthy donor PB (mean ± SEM) (Mann–Whitney U-test; ns).
, respectively). Statistical analysis was performed with Kruskal–Wallis and Dunn’s multiple comparisons test to compare epcoritamab with CD3xCtrl (*p < 0.05) and to compare epcoritamab-dependent cytotoxicity (subtracted with CD3xCTRL values) between subtypes (ns). B Ratio of CD4+ T helper cells (CD4+CD25−) to CD8+ T-cells in DLBCL, FL, and MCL samples (Kruskal–Wallis with Dunn’s multiple comparisons test; *p < 0.05, **p ≤ 0.01) (median ± interquartile range). C PD-1 expression (MFI values normalized to expression on T-cells of internal healthy donor control) on CD4+ T-cells of DLBCL, FL, including PD-1 bright and dim populations, and MCL (Kruskal–Wallis with Dunn’s multiple comparisons test; *p < 0.05, **p < 0.01, ***p < 0.001) (median ± interquartile range). D Spearman’s correlation between CD3:Target ratio and cytotoxicity mediated by epcoritamab (30 ng/mL) (r = 0.63; ****p < 0.0001). E Lymph node T-cells of B-NHL patients isolated by magnetic-activated cell-sorting (MACS) showed similar percentages B-cell lymphoma cell line (WSU-DLCL2 and Daudi, left to right) cytotoxicity as T-cells isolated from healthy donor PB (mean ± SEM) (Mann–Whitney U-test; ns).
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

