Ibrutinib and venetoclax target distinct subpopulations of CLL cells: implication for residual disease eradication
- PMID: 33602908
- PMCID: PMC7893066
- DOI: 10.1038/s41408-021-00429-z
Ibrutinib and venetoclax target distinct subpopulations of CLL cells: implication for residual disease eradication
Abstract
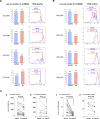
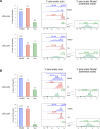
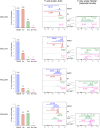
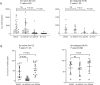
Ibrutinib inhibits Bruton tyrosine kinase while venetoclax is a specific inhibitor of the anti-apoptotic protein BCL2. Both drugs are highly effective as monotherapy against chronic lymphocytic leukemia (CLL), and clinical trials using the combination therapy have produced remarkable results in terms of rate of complete remission and frequency of undetectable minimal residual disease. However, the laboratory rationale behind the success of the drug combination is still lacking. A better understanding of how these two drugs synergize would eventually help develop other rational combination strategies. Using an ex vivo model that promotes CLL proliferation, we show that modeled ibrutinib proliferative responses, but not viability responses, correlate well with patients' actual clinical responses. Importantly, we demonstrate for the first time that ibrutinib and venetoclax act on distinct CLL subpopulations that have different proliferative capacities. While the dividing subpopulation of CLL responds to ibrutinib, the resting subpopulation preferentially responds to venetoclax. The combination of these targeted therapies effectively reduced both the resting and dividing subpopulations in most cases. Our laboratory findings help explain several clinical observations and contribute to the understanding of tumor dynamics. Additionally, our proliferation model may be used to identify novel drug combinations with the potential of eradicating residual disease.
Conflict of interest statement
Y.T., Speaker’s bureau for Novartis. S.M., Consultancy for AstraZeneca, Bioverativ, Genentech, Janssen, Kite and Pharmacyclics, Speaker’s bureau for AstraZeneca, Janssen and Pharmacyclics, and research funding from Abbvie, AstraZeneca, Beigene, Pharmacyclics, Juno and Novartis. N.K., advisory board for Pharmacyclics, Jessen and Seattle Genetics, and research funding from Bristol Meyer Squibb. Y.L.W., employment and stock ownership at Incyte Research Institute. All other authors declare no relevant conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

