Antibody mediated activation of natural killer cells in malaria exposed pregnant women
- PMID: 33602987
- PMCID: PMC7893158
- DOI: 10.1038/s41598-021-83093-4
Antibody mediated activation of natural killer cells in malaria exposed pregnant women
Abstract
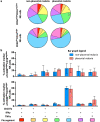
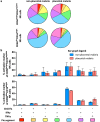
Immune effector responses against Plasmodium falciparum include antibody-mediated activation of innate immune cells, which can induce Fc effector functions, including antibody-dependent cellular cytotoxicity, and the secretion of cytokines and chemokines. These effector functions are regulated by the composition of immunoglobulin G (IgG) Fc N-linked glycans. However, a role for antibody-mediated natural killer (NK) cells activation or Fc N-linked glycans in pregnant women with malaria has not yet been established. Herein, we studied the capacity of IgG antibodies from pregnant women, with placental malaria or non-placental malaria, to induce NK cell activation in response to placental malaria-associated antigens DBL2 and DBL3. Antibody-mediated NK cell activation was observed in pregnant women with malaria, but no differences were associated with susceptibility to placental malaria. Elevated anti-inflammatory glycosylation patterns of IgG antibodies were observed in pregnant women with or without malaria infection, which were not seen in healthy non-pregnant controls. This suggests that pregnancy-associated anti-inflammatory Fc N-linked glycans may dampen the antibody-mediated activation of NK cells in pregnant women with malaria infection. Overall, although anti-inflammatory glycans and antibody-dependent NK cell activation were detected in pregnant women with malaria, a definitive role for these antibody features in protecting against placental malaria remains to be proven.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Fc-Afucosylation of VAR2CSA-Specific Immunoglobulin G and Clinical Immunity to Placental Plasmodium falciparum Malaria.J Infect Dis. 2025 Jun 2;231(5):e956-e965. doi: 10.1093/infdis/jiae529. J Infect Dis. 2025. PMID: 39585195 Free PMC article.
-
The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site.PLoS One. 2014 Feb 4;9(2):e88173. doi: 10.1371/journal.pone.0088173. eCollection 2014. PLoS One. 2014. PMID: 24505415 Free PMC article.
-
Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin.Emerg Infect Dis. 2015 May;21(5):813-23. doi: 10.3201/eid2105.141626. Emerg Infect Dis. 2015. PMID: 25898123 Free PMC article.
-
Malaria in pregnancy: pathogenesis and immunity.Lancet Infect Dis. 2007 Feb;7(2):105-17. doi: 10.1016/S1473-3099(07)70022-1. Lancet Infect Dis. 2007. PMID: 17251081 Review.
-
VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria.Front Immunol. 2021 Feb 9;11:624126. doi: 10.3389/fimmu.2020.624126. eCollection 2020. Front Immunol. 2021. PMID: 33633743 Free PMC article. Review.
Cited by
-
Impact of structural modifications of IgG antibodies on effector functions.Front Immunol. 2024 Jan 8;14:1304365. doi: 10.3389/fimmu.2023.1304365. eCollection 2023. Front Immunol. 2024. PMID: 38259472 Free PMC article. Review.
-
Association of the humoral immune response with the inflammatory profile in Plasmodium vivax infections in pregnant women.PLoS Negl Trop Dis. 2024 Nov 4;18(11):e0012636. doi: 10.1371/journal.pntd.0012636. eCollection 2024 Nov. PLoS Negl Trop Dis. 2024. PMID: 39495782 Free PMC article.
-
Parasitic infections during pregnancy in Gabon affect glycosylation patterns of maternal and child antibodies.Sci Rep. 2024 Dec 30;14(1):31879. doi: 10.1038/s41598-024-83366-8. Sci Rep. 2024. PMID: 39738418 Free PMC article.
-
Polyfunctional antibodies: a path towards precision vaccines for vulnerable populations.Front Immunol. 2023 Jun 27;14:1183727. doi: 10.3389/fimmu.2023.1183727. eCollection 2023. Front Immunol. 2023. PMID: 37600816 Free PMC article. Review.
-
PfEMP1 and var genes - Still of key importance in Plasmodium falciparum malaria pathogenesis and immunity.Adv Parasitol. 2024;125:53-103. doi: 10.1016/bs.apar.2024.02.001. Epub 2024 Mar 23. Adv Parasitol. 2024. PMID: 39095112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

