Disease progression modelling from preclinical Alzheimer's disease (AD) to AD dementia
- PMID: 33603015
- PMCID: PMC7893024
- DOI: 10.1038/s41598-021-83585-3
Disease progression modelling from preclinical Alzheimer's disease (AD) to AD dementia
Abstract
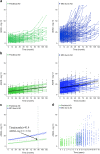
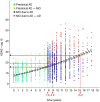
To characterize the course of Alzheimer's disease (AD) over a longer time interval, we aimed to construct a disease course model for the entire span of the disease using two separate cohorts ranging from preclinical AD to AD dementia. We modelled the progression course of 436 patients with AD continuum and investigated the effects of apolipoprotein E ε4 (APOE ε4) and sex on disease progression. To develop a model of progression from preclinical AD to AD dementia, we estimated Alzheimer's Disease Assessment Scale-Cognitive Subscale 13 (ADAS-cog 13) scores. When calculated as the median of ADAS-cog 13 scores for each cohort, the estimated time from preclinical AD to MCI due to AD was 7.8 years and preclinical AD to AD dementia was 15.2 years. ADAS-cog 13 scores deteriorated most rapidly in women APOE ε4 carriers and most slowly in men APOE ε4 non-carriers (p < 0.001). Our results suggest that disease progression modelling from preclinical AD to AD dementia may help clinicians to estimate where patients are in the disease course and provide information on variation in the disease course by sex and APOE ε4 status.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

