Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: long-term follow-up of the randomized controlled SORAML trial
- PMID: 33603142
- PMCID: PMC8410595
- DOI: 10.1038/s41375-021-01148-x
Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: long-term follow-up of the randomized controlled SORAML trial
Abstract
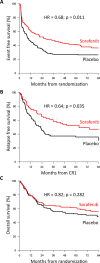
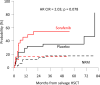
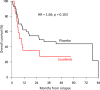
Early results of the randomized placebo-controlled SORAML trial showed that, in patients with newly diagnosed acute myeloid leukaemia (AML), sorafenib led to a significant improvement in event-free (EFS) and relapse-free survival (RFS). In order to describe second-line treatments and their implications on overall survival (OS), we performed a study after a median follow-up time of 78 months. Newly diagnosed fit AML patients aged ≤60 years received sorafenib (n = 134) or placebo (n = 133) in addition to standard chemotherapy and as maintenance treatment. The 5-year EFS was 41 versus 27% (HR 0.68; p = 0.011) and 5-year RFS was 53 versus 36% (HR 0.64; p = 0.035). Allogeneic stem cell transplantation (allo SCT) was performed in 88% of the relapsed patients. Four years after salvage allo SCT, the cumulative incidence of relapse was 54 versus 35%, and OS was 32 versus 50%. The 5-year OS from randomization in all study patients was 61 versus 53% (HR 0.82; p = 0.282). In conclusion, the addition of sorafenib to chemotherapy led to a significant prolongation of EFS and RFS. Although the OS benefit did not reach statistical significance, these results confirm the antileukaemic activity of sorafenib.
© 2021. The Author(s).
Conflict of interest statement
GE and CR received institutional funding for the study from Bayer HealthCare Germany. All other authors declare no competing financial interests.
Figures

References
-
- Klein M, Schermuly RT, Ellinghaus P, Milting H, Riedl B, Nikolova S, et al. Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation. 2008;118:2081–90. doi: 10.1161/CIRCULATIONAHA.108.779751. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

