Trends and associated maternal characteristics of antidiabetic medication use among pregnant women in South Korea
- PMID: 33603191
- PMCID: PMC7892865
- DOI: 10.1038/s41598-021-83808-7
Trends and associated maternal characteristics of antidiabetic medication use among pregnant women in South Korea
Abstract
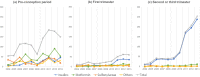
The prevalence of diabetes during pregnancy and the need for the treatment are increasing. We aimed to investigate antidiabetic medications (ADM) use among pregnant women and their characteristics. Using Korea's nationwide healthcare database, we included women aged 15-49 years with births during 2004-2013. The prevalence and secular trend of ADM use were assessed in 3 periods: pre-conception period, first trimester, and second/third trimesters. To compare maternal characteristics between pregnancies with and without ADM prescription, we used the χ2 or Fisher's exact test and Cochran-Armitage trend test. The prescription patterns analyzed by calendar year, age, insurance type, income, area, and medical institution. Of 81,559 pregnancies, 222 (0.27%) and 305 (0.37%) were exposed ADM during pre-conception and pregnancy periods, respectively. ADM prescriptions increased significantly by an 11.3-fold in second/third trimesters, while a 2.9-fold in first trimester. ADM use is more prevalent in women aged older and living in urban areas. Metformin was most used in the pre-conception period, while insulins were most during pregnancy. About 0.4% of women received ADM during pregnancy; a rate was lower than that in western countries. Non-recommended medications were more common in first trimester, which warrants pregnancy screening for women taking ADM.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical