Preferences of biobank participants for receiving actionable genomic test results: results of a recontacting study
- PMID: 33603197
- PMCID: PMC8194390
- DOI: 10.1038/s41436-021-01111-2
Preferences of biobank participants for receiving actionable genomic test results: results of a recontacting study
Abstract
Purpose: We sought to determine preferences of biobank participants whose samples were tested for clinically actionable variants but did not respond to an initial invitation to receive results.
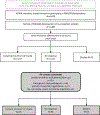
Methods: We recontacted a subsample of participants in the Kaiser Permanente Washington/University of Washington site of the Electronic Medical Records and Genomics (eMERGE3) Network. The subsample had provided broad consent for their samples to be used for research but had not responded to one initial mailed invitation to receive their results. We sent a letter from the principal investigators with phone outreach. If no contact was made, we sent a certified letter stating our assumption that participant had actively refused. We collected reasons for declining.
Results: We recontacted 123 participants. Response rate was 70.7% (n = 87). Of these, 62 (71.3%) declined the offer of returned results and 25 (28.7%) consented. The most common reasons provided for refusal included not wanting to know (n = 22) and concerns about insurability (n = 28).
Conclusion: Efforts to recontact biobank participants can yield high response. Though active refusal upon recontact was common, our data do not support assuming initial nonresponse to be refusal. Future research can work toward best practices for reconsenting, especially when clinically actionable results are possible.
Conflict of interest statement
CONFLICT OF INTEREST NOTIFICATION PAGE
The authors have no conflicts to declare.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources