Brain capillary pericytes exert a substantial but slow influence on blood flow
- PMID: 33603231
- PMCID: PMC8102366
- DOI: 10.1038/s41593-020-00793-2
Brain capillary pericytes exert a substantial but slow influence on blood flow
Abstract
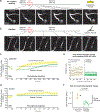
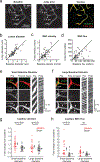
The majority of the brain's vasculature is composed of intricate capillary networks lined by capillary pericytes. However, it remains unclear whether capillary pericytes influence blood flow. Using two-photon microscopy to observe and manipulate brain capillary pericytes in vivo, we find that their optogenetic stimulation decreases lumen diameter and blood flow, but with slower kinetics than similar stimulation of mural cells on upstream pial and precapillary arterioles. This slow vasoconstriction was inhibited by the clinically used vasodilator fasudil, a Rho-kinase inhibitor that blocks contractile machinery. Capillary pericytes were also slower to constrict back to baseline following hypercapnia-induced dilation, and slower to dilate towards baseline following optogenetically induced vasoconstriction. Optical ablation of single capillary pericytes led to sustained local dilation and a doubling of blood cell flux selectively in capillaries lacking pericyte contact. These data indicate that capillary pericytes contribute to basal blood flow resistance and slow modulation of blood flow throughout the brain.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Figures





Comment in
-
A tense relationship between capillaries and pericytes.Nat Neurosci. 2021 May;24(5):615-617. doi: 10.1038/s41593-021-00853-1. Nat Neurosci. 2021. PMID: 33883740 No abstract available.
References
METHODS-ONLY REFERENCES
-
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

