Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial
- PMID: 33603241
- PMCID: PMC8818318
- DOI: 10.1038/s41591-020-01224-2
Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial
Abstract
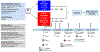
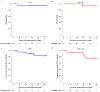
Ipilimumab improves clinical outcomes when combined with nivolumab in metastatic non-small cell lung cancer (NSCLC), but its efficacy and impact on the immune microenvironment in operable NSCLC remain unclear. We report the results of the phase 2 randomized NEOSTAR trial (NCT03158129) of neoadjuvant nivolumab or nivolumab + ipilimumab followed by surgery in 44 patients with operable NSCLC, using major pathologic response (MPR) as the primary endpoint. The MPR rate for each treatment arm was tested against historical controls of neoadjuvant chemotherapy. The nivolumab + ipilimumab arm met the prespecified primary endpoint threshold of 6 MPRs in 21 patients, achieving a 38% MPR rate (8/21). We observed a 22% MPR rate (5/23) in the nivolumab arm. In 37 patients resected on trial, nivolumab and nivolumab + ipilimumab produced MPR rates of 24% (5/21) and 50% (8/16), respectively. Compared with nivolumab, nivolumab + ipilimumab resulted in higher pathologic complete response rates (10% versus 38%), less viable tumor (median 50% versus 9%), and greater frequencies of effector, tissue-resident memory and effector memory T cells. Increased abundance of gut Ruminococcus and Akkermansia spp. was associated with MPR to dual therapy. Our data indicate that neoadjuvant nivolumab + ipilimumab-based therapy enhances pathologic responses, tumor immune infiltrates and immunologic memory, and merits further investigation in operable NSCLC.
Figures





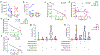




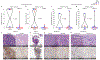

References
-
- Martin J, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 20, 1989–1995 (2002). - PubMed
-
- Pignon JP, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26, 3552–3559 (2008). - PubMed
Methods-only References
-
- Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10, 1–10 (1989). - PubMed
-
- Thall PF, Simon RM & Estey EH New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 14, 296–303 (1996). - PubMed
-
- Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247 (2009). - PubMed
-
- Woolson R a.C WR. Statistical Methods for the Analysis of Biomedical Data, (New York, 2002).
-
- Kaplan EL & Meier P Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 53, 457–481 (1958).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

