Synthesis and Structure-Property Relationships of Polyimide Covalent Organic Frameworks for Carbon Dioxide Capture and (Aqueous) Sodium-Ion Batteries
- PMID: 33603278
- PMCID: PMC7879495
- DOI: 10.1021/acs.chemmater.0c03218
Synthesis and Structure-Property Relationships of Polyimide Covalent Organic Frameworks for Carbon Dioxide Capture and (Aqueous) Sodium-Ion Batteries
Abstract
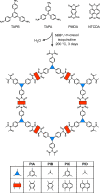
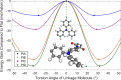
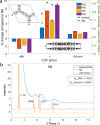
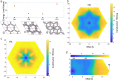
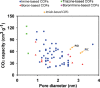
Covalent organic frameworks (COFs) are an emerging material family having several potential applications. Their porous framework and redox-active centers enable gas/ion adsorption, allowing them to function as safe, cheap, and tunable electrode materials in next-generation batteries, as well as CO2 adsorption materials for carbon-capture applications. Herein, we develop four polyimide COFs by combining aromatic triamines with aromatic dianhydrides and provide detailed structural and electrochemical characterization. Through density functional theory (DFT) calculations and powder X-ray diffraction, we achieve a detailed structural characterization, where DFT calculations reveal that the imide bonds prefer to form at an angle with one another, breaking the 2D symmetry, which shrinks the pore width and elongates the pore walls. The eclipsed perpendicular stacking is preferable, while sliding of the COF sheets is energetically accessible in a relatively flat energy landscape with a few metastable regions. We investigate the potential use of these COFs in CO2 adsorption and electrochemical applications. The adsorption and electrochemical properties are related to the structural and chemical characteristics of each COF, giving new insights for advanced material designs. For CO2 adsorption specifically, the two best performing COFs originated from the same triamine building block, which-in combination with force-field calculations-revealed unexpected structure-property relationships. Specific geometries provide a useful framework for Na-ion intercalation with retainable capacities and stable cycle life at a relatively high working potential (>1.5 V vs Na/Na+). Although this capacity is low compared to conventional inorganic Li-ion materials, we show as a proof of principle that these COFs are especially promising for sustainable, safe, and stable Na-aqueous batteries due to the combination of their working potentials and their insoluble nature in water.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Liang Y.; Tao Z.; Chen J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769. 10.1002/aenm.201100795. - DOI
-
- Song Z.; Zhou H. Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ. Sci. 2013, 6, 2280–2301. 10.1039/c3ee40709h. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
