The Impact of Muscarinic Receptor Antagonists on Airway Inflammation: A Systematic Review
- PMID: 33603353
- PMCID: PMC7886086
- DOI: 10.2147/COPD.S285867
The Impact of Muscarinic Receptor Antagonists on Airway Inflammation: A Systematic Review
Abstract
Long-acting muscarinic receptor antagonists (LAMAs) are the cornerstone for the treatment of chronic obstructive pulmonary disease (COPD); furthermore, tiotropium is approved as add-on therapy in severe asthmatic patients. Accumulating evidence suggests that LAMAs may modulate airway contractility and airway hyperresponsiveness not only by blocking muscarinic acetylcholine receptors (mAchRs) expressed on airway smooth muscle but also via anti-inflammatory mechanisms by blocking mAchRs expressed on inflammatory cells, submucosal glands, and epithelial cells. The aim of this systematic review, performed according to the PRISMA-P guidelines, was to provide a synthesis of the literature on the anti-inflammatory impact of muscarinic receptor antagonists in the airways. Most of the current evidence originates from studies on tiotropium, that demonstrated a reduction in synthesis and release of cytokines and chemokines, as well as the number of total and differential inflammatory cells, induced by different pro-inflammatory stimuli. Conversely, few data are currently available for aclidinium and glycopyrronium, whereas no studies on the potential anti-inflammatory effect of umeclidinium have been reported. Overall, a large body of evidence supports the beneficial impact of tiotropium against airway inflammation. Further well-designed randomized controlled trials are needed to better elucidate the anti-inflammatory mechanisms leading to the protective effect of LAMAs against exacerbations via identifying suitable biomarkers.
Keywords: LAMA; inflammation; muscarinic receptor antagonist; systematic review; tiotropium.
© 2021 Calzetta et al.
Conflict of interest statement
L.C. has participated as an advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis; received nonfinancial support from AstraZeneca; a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall; is or has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech; and his department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. A.Co. participated as a lecturer and speaker in scientific meetings and courses under the sponsorship of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Menarini Group and Zambon. B.L.R. has no conflict of interest to declare. M.M. has no conflict of interest to declare. A.Ch. received grants from Menarini and Astra Zeneca, and personal fee from Chiesi. P.R. participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, and her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici Novartis, and Zambon. The authors report no other potential conflicts of interest for this work.
Figures
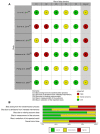
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of COPD – 2020 Report. 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-0.... Accessed July28, 2020.
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2020 Available from: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_.... Accessed July28, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

