The Prevalence of Chronic Obstructive Pulmonary Disease (COPD) and the Heterogeneity of Risk Factors in the Canadian Population: Results from the Canadian Obstructive Lung Disease (COLD) Study
- PMID: 33603357
- PMCID: PMC7886112
- DOI: 10.2147/COPD.S285338
The Prevalence of Chronic Obstructive Pulmonary Disease (COPD) and the Heterogeneity of Risk Factors in the Canadian Population: Results from the Canadian Obstructive Lung Disease (COLD) Study
Abstract
Purpose: To determine the spirometric-based prevalence of COPD across different regions in Canada and to evaluate the site heterogeneity of risk factors.
Patients and methods: In this cross-sectional, population-based study, random samples of non-institutionalized adults aged ≥40 years were generated by random digit dialling. Participants answered an interviewer-administered questionnaire and performed spirometry before and after bronchodilator administration. COPD was defined as post-bronchodilator FEV1/FVC <0.70 (fixed ratio, FR) and as FEV1/FVC <5th percentile (lower limits of normal, LLN). Separate logistic regression models were used to compute the risk (adjusted odds ratio, aOR) for COPD. I2 and Tau2 analyses were used to evaluate heterogeneity.
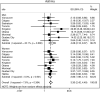
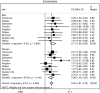
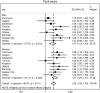
Results: Out of 5176 (95%) participants, 4893 (47% male with mean age 56.6 years (95% confidence interval, 56.0-57.2)) had spirometry that satisfied ATS criteria. The population prevalence of COPD was 16.2% (95% CI, 14.5-17.8) by FR and 11.2% (95% CI, 9.7-12.6) by LLN. Male predominance in prevalence was shown by FR but not by LLN criteria. Patient characteristics associated with an increased risk of COPD included: age (OR 1.56; 95% CI 1.33-1.84); history of physician-diagnosed asthma (OR 3.30; 95% CI 2.42-4.49); and childhood hospitalization for respiratory illness (OR 1.81; 95% CI 1.17-2.80). In terms of smoking-related risk factors, current smoking status had the highest odds ratio (OR 3.49; 95% CI 2.55-4.80). Variance in prevalence among sites was significantly reduced by adjusting for risk factors in Tau2 analyses. Higher odds of exposure for each risk factor was found in more severe COPD, suggesting that a higher risk could be linked to the development of severe disease.
Conclusion: This study reports the population prevalence of COPD in nine urban cities which collectively represent the majority of the Canadian population and demonstrates that heterogeneity in prevalence among sites is substantially explained by variation in associated risk factors for COPD.
Keywords: COPD; heterogeneity; prevalence.
© 2021 Leung et al.
Conflict of interest statement
Jean Bourbeau reports grants from CIHR, grants from Foundation of the MUHC, personal fees from Canadian Thoracic Society, personal fees from AstraZeneca, consultant/lecture for CHEST, advisor/lecture for Boehringer Ingelheim, Grifols, GlaxoSmithKline, and Novartis, lecture for Trudell, grants from Aerocrine, outside the submitted work. François Maltais reports grants from CanCOLD, during the conduct of the study; grants from GlaxoSmithKline, AstraZeneca, Sanofi, personal fees from GlaxoSmithKline, Boehringer Ingelheim, Grifols, Novartis, unrestricted grants paid to their institution from Novartis, Boehringer Ingelheim, Grifols, and financial participation in Oxynov, a company which is developing an oxygen delivery system, outside the submitted work. Darcy D Marciniuk reports grants from McGill University, during the conduct of the study; grants from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Canadian Health Infowayfor Healthcare Improvement, Lung Association of Saskatchewan, and Canadian Institute of Health Research, Grifols, Novartis, Sanofi, Saskatchewan Health Research Foundation, Schering-Plough, and is an employee of the University of Saskatchewan, outside the submitted work. Paul Hernandez reports personal fees from AstraZeneca, grants from Boehringer Ingelheim, Grifols, and Vertex, fees for medical advisory board from Actelion, GlaxoSmithKline, and Novartis, outside the submitted work. Wan C Tan reports grants from Canadian Institute of Heath Research, rgrants from GlaxoSmithKline Canada Ltd, nothing from AstraZeneca Canada Ltd., nothing from Boehringer-Ingelheim Canada Ltd, nothing from Novartis, nothing from Merck, nothing from Pfizer, Canada, and nothing from Nycomed, Canada, during the conduct of the study; personal fees from GlaxoSmithKline, Canada and AstraZeneca, Canada, outside the submitted work. The abstract of this paper was presented at the American Thoracic Society 2020 International Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the American Journal of Respiratory and Critical Care Medicine: https://doi.org/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A4566. This study complied with the Declaration of Helsinki.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

