Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R
- PMID: 33603537
- PMCID: PMC7873759
- DOI: 10.1016/j.jsps.2020.12.003
Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R
Abstract
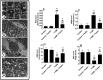
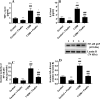
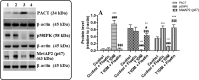
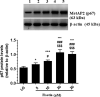
This study examined if the Fisetin against streptozotocin-induced diabetic cardiomyopathy (DC) in rats involves regulating cardiac metabolism and suppressing protein kinase R (PKR). Male rats were divided (12/groups) as control (non-diabetic), control + Fisetin, T1DM, and T1DM + Fisetin. Fisetin was administered orally at a final dose of 2.5 mg/kg for 12 weeks. In T1DM1-induced rats, Fisetin prevented heart and final body weights loss, lowered circulatory levels troponin I and creatinine kinase-MB (CK-MB), increased fasting insulin levels, and improved ventricular systolic and diastolic functions. It also preserved the structure of the cardiomyocytes and reduced oxidative stress, fibrosis, protein levels of transforming growth factor-β1 (TGF-β1), collagenase 1A, caspase-3, and the activation of JNK, p53, and p38 MAPK. In the control and diabetic rats, Fisetin attenuated fasting hyperglycaemia, the increases in glucose levels after the oral and insulin tolerance tests, and HOMA-IR. It also increased cardiac glucose oxidation by increasing the activity of private dehydrogenase (PDH), phosphofructokinase (PFK), protein levels of PPAR-α and suppressed cardiac inflammation by inhibiting NF-κB. These effects were associated with a reduction in the activity of PKR and subsequent increase in the activity of eeukaryotic initiation factor 2 (eIF2) with a parallel increase in protein levels of p67, a cellular inhibitor of PKR. In cultured cardiomyocytes, Fisetin, prevented high glucose (HG)-induced activation of PKR and reduction in p67, in a dose-dependent manner. However, the effect of Fisetin on PKR was diminished in LG and HG-treated cardiomyocytes with p67-siRNA. In conclusion, Fisetin protects against DC in rats by improving cardiac glucose metabolism and suppressing PKR.
Keywords: Diabetic cardiomyopathy; Fisetin; Glucose; Protein Kinase R; Rats.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Carvalho-Filho M.A., Carvalho B.M., Oliveira A.G., Guadagnini D., Ueno M., Dias M.M., Tsukumo D.M., Hirabara S.M., Reis L.F., Curi R., Carvalheira J.B., Saad M.J. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology. 2012;153(11):5261–5274. doi: 10.1210/en.2012-1400. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

