On the Suitability of Almond Shells for the Manufacture of a Natural Low-Cost Bioadsorbent to Remove Brilliant Green: Kinetics and Equilibrium Isotherms Study
- PMID: 33603573
- PMCID: PMC7868153
- DOI: 10.1155/2021/6659902
On the Suitability of Almond Shells for the Manufacture of a Natural Low-Cost Bioadsorbent to Remove Brilliant Green: Kinetics and Equilibrium Isotherms Study
Abstract
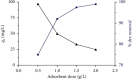
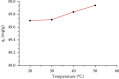
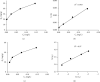
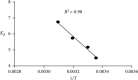
Almond production generates a large number of coproducts, but the farmer's interest mainly focuses on the nutritional and commercial aspects of the kernel for getting the best return from their harvests. Thus, almond coproducts such as almond shells that represent more than 70% of biomass remain underexplored. In this work, the suitability of almond shell powder (ASP) as a natural low-cost adsorbent was evaluated in the adsorption of brilliant green dye (BG), which is known as a chemical pollutant. Brunauer-Emmett-Teller (BET) method, for the determination of specific surface area, Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM) techniques were performed to characterize the ASP adsorbent. The batch adsorption kinetic study for the removal of BG dye was carried out by varying pH, temperature, initial concentration of the dye, bioadsorbent dose, and contact time. It was found that 98% of BG dye is removed under the following optimal experimental conditions: ASP bioadsorbent dose of 1 g/L at T = 25°C, pH = 6.8, and C 0 = 1 g/L, which proves that ASP can be used as an excellent low-cost bioadsorbent for the removal of BG dye from wastewater. The experimental isotherm data were analyzed using Freundlich and Langmuir models. The results show the best correlation with single-layer adsorption, and the adsorption kinetics seems to follow a pseudo-second-order model.
Copyright © 2021 R. Melhaoui et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Bhattacharya D., Ghoshal D., Mondal D., et al. Visible light driven degradation of brilliant green dye using titanium based ternary metal oxide photocatalyst. Results in Physics. 2019;12:1850–1858. doi: 10.1016/j.rinp.2019.01.065. - DOI
-
- Ahmadi K., Ghaedi M., Ansari A. Comparison of nickel doped Zinc Sulfide and/or palladium nanoparticle loaded on activated carbon as efficient adsorbents for kinetic and equilibrium study of removal of Congo Red dye. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;136:1441–1449. doi: 10.1016/j.saa.2014.10.034. - DOI - PubMed
-
- Brüschweiler B. J., Küng S., Bürgi D., Muralt L., Nyfeler E. Identification of non-regulated aromatic amines of toxicological concern which can be cleaved from azo dyes used in clothing textiles. Regulatory Toxicology and Pharmacology. 2014;69(2):263–272. doi: 10.1016/j.yrtph.2014.04.011. - DOI - PubMed
-
- Boumchita S., Lahrichi A., Benjelloun Y., Lairini S., Nenov V., Zerrouq F. Removal of cationic dye from aqueous solution by a food waste: potato peel. Journal of Materials and Environmental Science. 2016;7:73–84.
LinkOut - more resources
Full Text Sources
Other Literature Sources

