Clinical consequences of the presence of anti-RNA Pol III antibodies in systemic sclerosis
- PMID: 33603608
- PMCID: PMC7874867
- DOI: 10.5114/ada.2020.102107
Clinical consequences of the presence of anti-RNA Pol III antibodies in systemic sclerosis
Abstract
Introduction: Anti-RNA polymerase III (a-RNA Pol III) antibodies are marker antibodies in patients with systemic sclerosis (SSc).
Aim: To assess the prevalence of a-RNA Pol III in patients with SSc and to identify the differences in the disease picture in SSc patients with and without a-RNA Pol III antibodies.
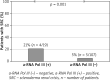
Material and methods: The study was performed in 126 SSc patients. The subtype of SSc, incidence of internal organ involvement, malignancy, death and serological profiles were determined in the entire group. The study groups were studied according to the presence of antibodies by applying the commercial test - EUROLINE SSc Profile. Due to the presence of a-RNA Pol III, patients were divided into two groups: the a-RNA Pol III (+) SSc group of 19 patients and the a-RNA Pol III (-) SSc group of 107 patients.
Results: A-RNA Pol III were present in 19/126 patients with SSc (15%), 13/19 (68.4%) patients had no other SSc marker antibodies. A-RNA Pol III were more common in patients with diffuse cutaneous SSc (p = 0.049). We showed a significant positive association between a-RNA Pol III and occurrence of malignancy (p = 0.007), scleroderma renal crisis (p = 0.001) and decreased DLCO (p = 0.007).
Conclusions: Anti-a-RNA Pol III antibodies are common in patients with SSc, particularly with a diffuse subtype. In more than 50% of patients with a-RNA Pol III antibodies, they may be present as the sole marker of antibodies. In SSc, a-RNA Pol III antibodies are frequently associated with malignancy occurrence, kidney and lung involvement.
Keywords: anti-RNA Pol III antibodies; malignancies; systemic sclerosis.
Copyright: © 2021 Termedia Sp. z o. o.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jeong S, Hwang H, Roh J, et al. Evaluation of an automated screening assay, compared to indirect immunofluorescence, an extractable nuclear antigen assay, and a line immunoassay in a large cohort of asian patients with antinuclear antibody-associated rheumatoid diseases: a multicenter retrospective study. J Immunol Res. 2018;2018 doi: 10.1155/2018/9094217. - DOI - PMC - PubMed
-
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–55. - PubMed
-
- Kuwana M. Circulating anti-nuclear antibodies in systemic sclerosis: utility in diagnosis and disease subsetting. J Nippon Med Sch. 2017;84:56–63. - PubMed
-
- Satiago M, Baron M, Burlingame RW, et al. Antibodies to RNA polymerase III in systemic sclerosis as detected by an Elisa. J Rheumatol. 2007;34:1528–34. - PubMed
-
- Meyer O, De Chaisemartin L, Nicaise R, et al. Anti-RNA polymerase III antibody prevalence and associated clinical manifestations in a large series of French patients with systemic sclerosis: a-cross-sectional study. J Rheumatol. 2010;37:125–30. - PubMed
LinkOut - more resources
Full Text Sources