Recent Advances in Neural Circuits for Taste Perception in Hunger
- PMID: 33603648
- PMCID: PMC7884326
- DOI: 10.3389/fncir.2021.609824
Recent Advances in Neural Circuits for Taste Perception in Hunger
Abstract
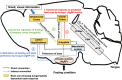
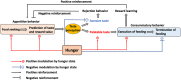
Feeding is essential for survival and taste greatly influences our feeding behaviors. Palatable tastes such as sweet trigger feeding as a symbol of a calorie-rich diet containing sugar or proteins, while unpalatable tastes such as bitter terminate further consumption as a warning against ingestion of harmful substances. Therefore, taste is considered a criterion to distinguish whether food is edible. However, perception of taste is also modulated by physiological changes associated with internal states such as hunger or satiety. Empirically, during hunger state, humans find ordinary food more attractive and feel less aversion to food they usually dislike. Although functional magnetic resonance imaging studies performed in primates and in humans have indicated that some brain areas show state-dependent response to tastes, the mechanisms of how the brain senses tastes during different internal states are poorly understood. Recently, using newly developed molecular and genetic tools as well as in vivo imaging, researchers have identified many specific neuronal populations or neural circuits regulating feeding behaviors and taste perception process in the central nervous system. These studies could help us understand the interplay between homeostatic regulation of energy and taste perception to guide proper feeding behaviors.
Keywords: appetitive and consummatory behaviors; hunger; neural circuit; satiety; taste.
Copyright © 2021 Fu, Minokoshi and Nakajima.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Recent advances in the characterization of genetically defined neurons that regulate internal-state-dependent taste modification in mice.Physiol Rep. 2024 Nov;12(21):e70106. doi: 10.14814/phy2.70106. Physiol Rep. 2024. PMID: 39523492 Free PMC article. Review.
-
Neural insights into sweet taste transduction and hunger-induced taste modification in mice.Biosci Biotechnol Biochem. 2022 Oct 20;86(11):1485-1489. doi: 10.1093/bbb/zbac142. Biosci Biotechnol Biochem. 2022. PMID: 35998309 Review.
-
Hunger state affects both olfactory abilities and gustatory sensitivity.Eur Arch Otorhinolaryngol. 2016 Jul;273(7):1637-41. doi: 10.1007/s00405-015-3589-6. Epub 2015 Mar 6. Eur Arch Otorhinolaryngol. 2016. PMID: 25744049
-
Tasting calories differentially affects brain activation during hunger and satiety.Behav Brain Res. 2015 Feb 15;279:139-47. doi: 10.1016/j.bbr.2014.11.019. Epub 2014 Nov 20. Behav Brain Res. 2015. PMID: 25449847
-
Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses.Elife. 2019 Jun 17;8:e47677. doi: 10.7554/eLife.47677. Elife. 2019. PMID: 31205005 Free PMC article.
Cited by
-
Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding.Nutrients. 2022 Dec 4;14(23):5161. doi: 10.3390/nu14235161. Nutrients. 2022. PMID: 36501191 Free PMC article.
-
Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior.Nutrients. 2024 Aug 12;16(16):2661. doi: 10.3390/nu16162661. Nutrients. 2024. PMID: 39203798 Free PMC article.
-
The impact of cognitive distraction on gustatory perception in volunteers with obesity.Sci Rep. 2024 Jun 20;14(1):14268. doi: 10.1038/s41598-024-64722-0. Sci Rep. 2024. PMID: 38902292 Free PMC article.
-
Sensory detection of female olfactory cues as a central regulator of energy metabolism and body weight in male mice.iScience. 2023 Mar 20;26(4):106455. doi: 10.1016/j.isci.2023.106455. eCollection 2023 Apr 21. iScience. 2023. PMID: 37020965 Free PMC article.
-
An all 2D bio-inspired gustatory circuit for mimicking physiology and psychology of feeding behavior.Nat Commun. 2023 Sep 27;14(1):6021. doi: 10.1038/s41467-023-41046-7. Nat Commun. 2023. PMID: 37758750 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

