MiR-143-3p Inhibits Osteogenic Differentiation of Human Periodontal Ligament Cells by Targeting KLF5 and Inactivating the Wnt/β-Catenin Pathway
- PMID: 33603676
- PMCID: PMC7884451
- DOI: 10.3389/fphys.2020.606967
MiR-143-3p Inhibits Osteogenic Differentiation of Human Periodontal Ligament Cells by Targeting KLF5 and Inactivating the Wnt/β-Catenin Pathway
Abstract
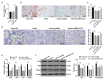
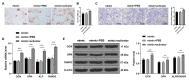
Human periodontal ligament cells (hPDLCs) play a vital role in cell regeneration and tissue repair with multi-directional differentiation potential. microRNAs (miRs) are implicated in the osteogenesis of hPDLCs. This study explored the mechanism of miR-143-3p in osteogenesis of hPDLCs. Osteogenic differentiation of isolated hPDLCs was induced. KLF5 expression during osteogenic differentiation of hPDLCs was detected and then silenced in hPDLCs. Binding relationship between KLF5 and miR-143-3p was predicted and verified. hPDLCs were treated with miR-143-3p mimic or overexpressing KLF5, and then osteogenic specific markers and mineralized nodules were measured. The key factors of the Wnt/β-catenin pathway during osteogenesis of hPDLCs were measured. KLF5 expression was upregulated during osteogenesis of hPDLCs. KLF5 silencing or miR-143-3p mimic reduced osteogenic specific markers and mineralized nodules. Overexpression of KLF5 could reverse the inhibitory effect of miR-143-3p on osteogenic differentiation. miR-143-3p mimic and KLF5 silencing inactivated the Wnt/β-catenin pathway. Activation of the Wnt/β-catenin pathway reversed the repression effect of miR-143-3p mimic on osteogenesis of hPDLCs. In conclusion, miR-143-3p inhibited osteogenic differentiation of hPDLCs by targeting KLF5 and inactivating the Wnt/β-catenin pathway.
Keywords: KLF5; Wnt/β-catenin pathway; human periodontal ligament cells; microRNA-143-3p; osteogenic differentiation.
Copyright © 2021 Wangzhou, Lai, Lu, Fu, Liu, Liang, Tan, Li and Hao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

