Neuromonitoring in Neonatal-Onset Epileptic Encephalopathies
- PMID: 33603712
- PMCID: PMC7884638
- DOI: 10.3389/fneur.2021.623625
Neuromonitoring in Neonatal-Onset Epileptic Encephalopathies
Abstract
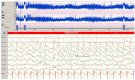
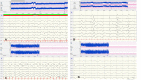
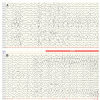
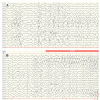
Considering the wide spectrum of etiologies of neonatal-onset epileptic encephalopathies (EE) and their unfavorable consequences for neurodevelopmental prognoses, neuromonitoring at-risk neonates is increasingly important. EEG is highly sensitive for early identification of electrographic seizures and abnormal background activity. Amplitude-integrated EEG (aEEG) is recommended as a useful bedside monitoring method but as a complementary tool because of methodical limitations. It is of special significance in monitoring neonates with acute symptomatic as well as structural, metabolic and genetic neonatal-onset EE, being at high risk of electrographic-only and prolonged seizures. EEG/aEEG monitoring is established as an adjunctive tool to confirm perinatal hypoxic-ischemic encephalopathy (HIE). In neonates with HIE undergoing therapeutic hypothermia, burst suppression pattern is associated with good outcomes in about 40% of the patients. The prognostic specificity of EEG/aEEG is lower compared to cMRI. As infants with HIE may develop seizures after cessation of hypothermia, recording for at least 24 h after the last seizure is recommended. Progress in the identification of genetic etiology of neonatal EE constantly increases. However, presently, no specific EEG changes indicative of a genetic variant have been characterized, except for individual variants associated with typical EEG patterns (e.g., KCNQ2, KCNT1). Long-term monitoring studies are necessary to define and classify electro-clinical patterns of neonatal-onset EE.
Keywords: electroencephalopgraphy; genetic epilepsy; metabolic epilepsy; neonatal brain; suppression burst.
Copyright © 2021 Trollmann.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

