Potential Application of T-Follicular Regulatory Cell Therapy in Transplantation
- PMID: 33603742
- PMCID: PMC7884443
- DOI: 10.3389/fimmu.2020.612848
Potential Application of T-Follicular Regulatory Cell Therapy in Transplantation
Abstract
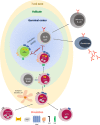
Regulatory T cells (Tregs) constitute a small proportion of circulating CD4+ T cells that function to maintain homeostasis and prevent autoimmunity. In light of their powerful immunosuppressive and tolerance-promoting properties, Tregs have become an interesting potential candidate for therapeutic use in conditions such as solid organ transplant or to treat autoimmune and inflammatory conditions. Clinical studies have demonstrated the safety of polyclonally expanded Tregs in graft-versus-host disease, type 1 diabetes, and more recently in renal and liver transplantation. However, Tregs are heterogenous. Recent insights indicate that only a small proportion of Tregs, called T follicular regulatory cells (Tfr) regulate interactions between B cells and T follicular helper (Tfh) cells within the germinal center. Tfr have been mainly described in mouse models due to the challenges of sampling secondary lymphoid organs in humans. However, emerging human studies, characterize Tfr as being CD4+CD25+FOXP3+CXCR5+ cells with different levels of PD-1 and ICOS expression depending on their localization, in the blood or the germinal center. The exact role they play in transplantation remains to be elucidated. However, given the potential ability of these cells to modulate antibody responses to allo-antigens, there is great interest in exploring translational applications in situations where B cell responses need to be regulated. Here, we review the current knowledge of Tfr and the role they play focusing on human diseases and transplantation. We also discuss the potential future applications of Tfr therapy in transplantation and examine the evidence for a role of Tfr in antibody production, acute and chronic rejection and tertiary lymphoid organs. Furthermore, the potential impact of immunosuppression on Tfr will be explored. Based on preclinical research, we will analyse the rationale of Tfr therapy in solid organ transplantation and summarize the different challenges to be overcome before Tfr therapy can be implemented into clinical practice.
Keywords: T-follicular regulatory cell; cell therapy; immunosuppression; regulatory T cell; transplantation.
Copyright © 2021 Dudreuilh, Basu, Scottà, Dorling and Lombardi.
Conflict of interest statement
GL is co-Founder of Quell Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
T follicular helper cell-mediated IL-21 production suppresses FOXP3 expression of T follicular regulatory-like cells in diffuse large B cell lymphoma patients.Hum Immunol. 2020 Aug;81(8):452-459. doi: 10.1016/j.humimm.2020.05.008. Epub 2020 Jun 10. Hum Immunol. 2020. PMID: 32534760
-
IL-21 Receptor Blockade Shifts the Follicular T Cell Balance and Reduces De Novo Donor-Specific Antibody Generation.Front Immunol. 2021 Apr 9;12:661580. doi: 10.3389/fimmu.2021.661580. eCollection 2021. Front Immunol. 2021. PMID: 33897706 Free PMC article.
-
Follicular helper and follicular regulatory T cell subset imbalance is associated with higher activated B cells and abnormal autoantibody production in primary anti-phospholipid syndrome patients.Clin Exp Immunol. 2021 Nov;206(2):141-152. doi: 10.1111/cei.13647. Epub 2021 Aug 22. Clin Exp Immunol. 2021. PMID: 34309827 Free PMC article. Clinical Trial.
-
Frontiers of Autoantibodies in Autoimmune Disorders: Crosstalk Between Tfh/Tfr and Regulatory B Cells.Front Immunol. 2021 Mar 26;12:641013. doi: 10.3389/fimmu.2021.641013. eCollection 2021. Front Immunol. 2021. PMID: 33841422 Free PMC article. Review.
-
Interaction Between Tfh/Tfr Ratio and Regulatory B Cell in Autoimmune Diseases.Iran J Immunol. 2025 Mar 30;22(1):1-12. doi: 10.22034/iji.2025.103848.2859. Iran J Immunol. 2025. PMID: 40057841 Review.
Cited by
-
Activation and Regulation of Indirect Alloresponses in Transplanted Patients With Donor Specific Antibodies and Chronic Rejection.Transpl Int. 2024 Aug 20;37:13196. doi: 10.3389/ti.2024.13196. eCollection 2024. Transpl Int. 2024. PMID: 39228658 Free PMC article. Review.
-
Disordered Balance of T-Cell Subsets in Arterial Tertiary Lymphoid Organs in Immunoglobulin G4-Related Vascular Disease.J Am Heart Assoc. 2023 Dec 19;12(24):e030356. doi: 10.1161/JAHA.123.030356. Epub 2023 Dec 8. J Am Heart Assoc. 2023. PMID: 38063185 Free PMC article.
-
CD4+CD25+ T regulatory cells in renal transplantation.Front Immunol. 2022 Nov 8;13:1017683. doi: 10.3389/fimmu.2022.1017683. eCollection 2022. Front Immunol. 2022. PMID: 36426347 Free PMC article. Review.
-
Romidepsin (FK228) improves the survival of allogeneic skin grafts through downregulating the production of donor-specific antibody via suppressing the IRE1α-XBP1 pathway.J Zhejiang Univ Sci B. 2022 May 15;23(5):392-406. doi: 10.1631/jzus.B2100780. J Zhejiang Univ Sci B. 2022. PMID: 35557040 Free PMC article.
-
Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19-BNT162b2 mRNA Vaccination.Viruses. 2022 Jul 2;14(7):1470. doi: 10.3390/v14071470. Viruses. 2022. PMID: 35891450 Free PMC article.
References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol (1995) 155(3):1151–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

