The Role of T Cell Receptor Signaling in the Development of Type 1 Diabetes
- PMID: 33603744
- PMCID: PMC7884625
- DOI: 10.3389/fimmu.2020.615371
The Role of T Cell Receptor Signaling in the Development of Type 1 Diabetes
Abstract
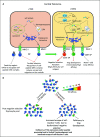
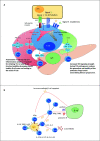
T cell receptor (TCR) signaling influences multiple aspects of CD4+ and CD8+ T cell immunobiology including thymic development, peripheral homeostasis, effector subset differentiation/function, and memory formation. Additional T cell signaling cues triggered by co-stimulatory molecules and cytokines also affect TCR signaling duration, as well as accessory pathways that further shape a T cell response. Type 1 diabetes (T1D) is a T cell-driven autoimmune disease targeting the insulin producing β cells in the pancreas. Evidence indicates that dysregulated TCR signaling events in T1D impact the efficacy of central and peripheral tolerance-inducing mechanisms. In this review, we will discuss how the strength and nature of TCR signaling events influence the development of self-reactive T cells and drive the progression of T1D through effects on T cell gene expression, lineage commitment, and maintenance of pathogenic anti-self T cell effector function.
Keywords: T cell differentiation; T cell receptor (TCR) signaling; autoimmunity; diabetes; immunoregulation.
Copyright © 2021 Clark, Kroger, Ke and Tisch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Eisenbarth GS. Type 1 diabetes: molecular, cellular and clinical immunology. Adv Exp Med Biol (2004) 552:306–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

