T-Bet Controls Cellularity of Intestinal Group 3 Innate Lymphoid Cells
- PMID: 33603753
- PMCID: PMC7884460
- DOI: 10.3389/fimmu.2020.623324
T-Bet Controls Cellularity of Intestinal Group 3 Innate Lymphoid Cells
Abstract
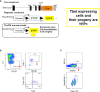
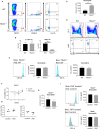
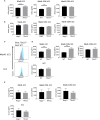
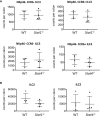
Innate lymphoid cells (ILC) play a significant immunological role at mucosal surfaces such as the intestine. T-bet-expressing group 1 innate lymphoid cells (ILC1) are believed to play a substantial role in inflammatory bowel disease (IBD). However, a role of T-bet-negative ILC3 in driving colitis has also been suggested in mouse models questioning T-bet as a critical factor for IBD. We report here that T-bet deficient mice had a greater cellularity of NKp46-negative ILC3 correlating with enhanced expression of RORγt and IL-7R, but independent of signaling through STAT1 or STAT4. We observed enhanced neutrophilia in the colonic lamina propria (cLP) of these animals, however, we did not detect a greater risk of T-bet-deficient mice to develop spontaneous colitis. Furthermore, by utilizing an in vivo fate-mapping approach, we identified a population of T-bet-positive precursors in NKp46-negative ILC3s. These data suggest that T-bet controls ILC3 cellularity, but does do not drive a pathogenic role of ILC3 in mice with a conventional specific pathogen-free microbiota.
Keywords: ILCs; T-bet; innate lymphoid cells; intestinal inflammation; mucosal homeostasis.
Copyright © 2021 Schroeder, Meissl, Hromadová, Lo, Neves, Howard, Helmby, Powell, Strobl and Lord.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

