Impaired Cellular Immunity to SARS-CoV-2 in Severe COVID-19 Patients
- PMID: 33603759
- PMCID: PMC7884325
- DOI: 10.3389/fimmu.2021.603563
Impaired Cellular Immunity to SARS-CoV-2 in Severe COVID-19 Patients
Abstract
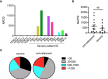
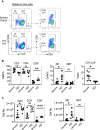
The high infection rate and rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) make it a world-wide pandemic. Individuals infected by the virus exhibited different degrees of symptoms, and most convalescent individuals have been shown to develop both cellular and humoral immune responses. However, virus-specific adaptive immune responses in severe patients during acute phase have not been thoroughly studied. Here, we found that in a group of COVID-19 patients with acute respiratory distress syndrome (ARDS) during hospitalization, most of them mounted SARS-CoV-2-specific antibody responses, including neutralizing antibodies. However, compared to healthy controls, the percentages and absolute numbers of both NK cells and CD8+ T cells were significantly reduced, with decreased IFNγ expression in CD4+ T cells in peripheral blood from severe patients. Most notably, their peripheral blood lymphocytes failed in producing IFNγ against viral proteins. Thus, severe COVID-19 patients at acute infection stage developed SARS-CoV-2-specific antibody responses but were impaired in cellular immunity, which emphasizes on the role of cellular immunity in COVID-19.
Keywords: SARS-CoV-2; T cells; acute respiratory distress syndrome; adaptive immunity; interferon gamma; neutralization antibody.
Copyright © 2021 Ni, Cheng, Feng, Zhao, Liu, Ye, Ye, Zhu, Li, Wang, Shao, Deng, Wei, Chen, Qin, Wang, Li, Zeng and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Di Pierro F, Bertuccioli A, Cavecchia I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol Dietol (2020) 66(2):172–176. 10.23736/S1121-421X.20.02697-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

