Evaluation of Medicine Exposure During Pregnancy at a Tertiary Center of an Indian State
- PMID: 33603908
- PMCID: PMC7879351
- DOI: 10.26574/maedica.2020.15.4.503
Evaluation of Medicine Exposure During Pregnancy at a Tertiary Center of an Indian State
Abstract
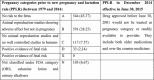
Context:Prescribing drugs in pregnancy is a challenging approach for doctors. Objective:To evaluate drugs used in pregnancy. Method:A prospective, cross-sectional, descriptive study was carried out by collecting and evaluating prescriptions on various parameters. Results:More than 50% of antenatal care attendees belonged to the 18-24 age group, and 102 (41.46%) were primigravidae. The main presenting complaints were abdominal pain (25.16%), followed by nausea and vomiting (22.60%) and fever (11.14%); the maximum number of visits to hospital was seen in the first trimester (40.53%), followed by the third trimester (38.42%). It was observed that 25.78% of prescriptions did not contain any medicine. The average number of prescribed medicines was 2.32, with the lowest in the first trimester (1.77) and the highest in the second trimester (2.78). It was noticed that 74.11% and 71.26% of all prescribed medicines were from essential medicine list and generics, respectively. Of all prescribed drugs, 11.52% were antimicrobials, and 4.11% injectable dosage forms. Vitamins and minerals were the preferred prescribed medicines (34.82%), followed by antimicrobial agents (11.52%) and doxylamine plus pyridoxine (10.16%). Also, doctors who made the drug choice during antenatal visits were more confident in evidence-based safety as per New Pregnancy and Lactation Rule (PPLR); 45.37% of drugs were prescribed from category A, followed by 38.25% from category B and none from group X. Conclusion:Doctors were concerned about prescribing safer drugs in pregnancy and were more confident in evidence-based medication.
Figures







Similar articles
-
Medication utilization pattern for management of pregnancy complications: a study in Western Nepal.BMC Pregnancy Childbirth. 2016 Sep 20;16:272. doi: 10.1186/s12884-016-1068-8. BMC Pregnancy Childbirth. 2016. PMID: 27644958 Free PMC article.
-
Drug use evaluation in pregnant women attending antenatal care in Shashemene Referral Hospital, Oromia Regional State, Ethiopia.SAGE Open Med. 2020 Sep 18;8:2050312120959178. doi: 10.1177/2050312120959178. eCollection 2020. SAGE Open Med. 2020. PMID: 32999721 Free PMC article.
-
Assessment of Antimicrobial Prescribing Pattern in the Outpatient Department of Ophthalmology in a Tertiary Care Hospital of Western Uttar Pradesh, India.Nepal J Ophthalmol. 2018 Jul;10(20):130-138. doi: 10.3126/nepjoph.v10i2.23014. Nepal J Ophthalmol. 2018. PMID: 31056555
-
Nausea and vomiting in pregnancy: a review of the pathology and compounding opportunities.Int J Pharm Compd. 2013 Mar-Apr;17(2):113-23. Int J Pharm Compd. 2013. PMID: 23696171 Review.
-
The pharmacologic management of nausea and vomiting of pregnancy.J Fam Pract. 2014 Feb;63(2 Suppl):S31-7. J Fam Pract. 2014. PMID: 24527483 Review.
References
-
- Locktich G. Clinical biochemistry of pregnancy. Crit Rev Clin Lab Sci. 1997;34:6. - PubMed
-
- Yankowitz J, Niebyl JR, editors. Drug therapy in pregnancy. 3rd ed. Philadelphia Lippincott William Wilkins. 2001.
-
- Sharma R, Kapoor B, Verma U. Drug utilization pattern during pregnancy in North India. J Med Sci. 2006;60:277–287. - PubMed
-
- Porter RS, editor. The Merck Manuals. Online Medical Library. Whitehouse Station: Merck Research Lab. 2004.
Publication types
LinkOut - more resources
Full Text Sources
