Development of a Hematopoietic Prostaglandin D Synthase-Degradation Inducer
- PMID: 33603969
- PMCID: PMC7883460
- DOI: 10.1021/acsmedchemlett.0c00605
Development of a Hematopoietic Prostaglandin D Synthase-Degradation Inducer
Abstract
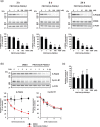
Although hematopoietic prostaglandin D synthase (H-PGDS) is an attractive target for treatment of a variety of diseases, including allergic diseases and Duchenne muscular dystrophy, no H-PGDS inhibitors have yet been approved for treatment of these diseases. Therefore, the development of novel agents having other modes of action to modulate the activity of H-PGDS is required. In this study, a chimeric small molecule that degrades H-PGDS via the ubiquitin-proteasome system, PROTAC(H-PGDS)-1, was developed. PROTAC(H-PGDS)-1 is composed of two ligands, TFC-007 (that binds to H-PGDS) and pomalidomide (that binds to cereblon). PROTAC(H-PGDS)-1 showed potent activity in the degradation of H-PGDS protein via the ubiquitin-proteasome system and in the suppression of prostaglandin D2 (PGD2) production. Notably, PROTAC(H-PGDS)-1 showed sustained suppression of PGD2 production after the drug removal, whereas PGD2 production recovered following removal of TFC-007. Thus, the H-PGDS degrader-PROTAC(H-PGDS)-1-is expected to be useful in biological research and clinical therapies.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Lewis R. A.; Soter N. A.; Diamond P. T.; Austen K. F.; Oates J. A.; Roberts L. J. Prostaglandin D2 Generation after Activation of Rat and Human Mast Cells with Anti-IgE. J. Immunol. 1982, 129, 1627–1631. - PubMed
-
- Matsuoka T.; Hirata M.; Tanaka H.; Takahashi Y.; Murata T.; Kabashima K.; Sugimoto Y.; Kobayashi T.; Ushikubi F.; Aze Y.; Eguchi N.; Urade Y.; Yoshida N.; Kimura K.; Mizoguchi A.; Honda Y.; Nagai H.; Narumiya S. Prostaglandin D2 as a Mediator of Allergic Asthma. Science 2000, 287, 2013–2017. 10.1126/science.287.5460.2013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

