Non-invasive acoustic fabrication methods to enhance collagen hydrogel bioactivity
- PMID: 33604057
- PMCID: PMC7888985
- DOI: 10.1088/2053-1591/ab597a
Non-invasive acoustic fabrication methods to enhance collagen hydrogel bioactivity
Abstract
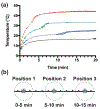
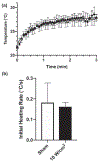
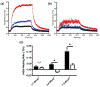
Much attention has focused recently on utilizing components of the extracellular matrix (ECM) as natural building blocks for a variety of tissue engineering applications and regenerative medicine therapies. Consequently, new fabrication methods are being sought to enable molecular control over the structural characteristics of ECM molecules in order to improve their biological function. Exposing soluble collagen to acoustic forces associated with ultrasound propagation produces localized variations in collagen microfiber organization that in turn, promote cell behaviors essential for tissue regeneration, including cell migration and matrix remodeling. In the present study, mechanisms by which ultrasound interacts with polymerizing collagen to produce functional changes in collagen microstructure were investigated. The rate of collagen polymerization was manipulated by adjusting the pH of collagen solutions and the temperature at which gels were polymerized. Results demonstrate that the phase transition of type I collagen from fluid to gel triggered a simultaneous increase in acoustic absorption. This phase transition of collagen involves the lateral growth of early-stage collagen microfibrils and importantly, corresponded to a defined period of time during which exposure to ultrasound introduced both structural and functional changes to the resultant collagen hydrogels. Together, these experiments isolated a critical window in the collagen fiber assembly process during which mechanical forces associated with ultrasound propagation are effective in producing structural changes that underlie the ability of acoustically-modified collagen hydrogels to stimulate cell migration. These results demonstrate that changes in material properties associated with collagen polymerization are a fundamental component of the mechanism by which acoustic forces modify collagen biomaterials to enhance biological function.
Keywords: acoustics; biofabrication; collagen; tissue engineering; ultrasound.
Conflict of interest statement
Declarations of interest none
Figures

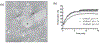
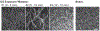

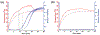
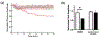
References
-
- Langer R and Vacanti JP 1993. Tissue engineering Science 260 920–6 - PubMed
-
- Abou Neel EA, Bozec L, Knowles JC, Syed O, Mudera V, Day R and Keun Hyun J 2013. Collagen—emerging collagen based therapies hit the patient Adv. Drug Delivery Rev 65 429–56 - PubMed
-
- Kadler KE, Baldock C, Bella JandBoot-Handford RP 2007. Collagens at a glance J. Cell Sci 120 1955–8 - PubMed
-
- Cen L, Liu W, Cui L, Zhang W and Cao Y 2008. Collagen tissue engineering: development of novel biomaterials and applications Pediatr Res 63 492–6 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
