Polycyclic aromatic hydrocarbons in yerba mate (Ilex paraguariensis) infusions and probabilistic risk assessment of exposure
- PMID: 33604246
- PMCID: PMC7875766
- DOI: 10.1016/j.toxrep.2021.01.017
Polycyclic aromatic hydrocarbons in yerba mate (Ilex paraguariensis) infusions and probabilistic risk assessment of exposure
Abstract
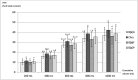
The aim of this study was to assess the risk of exposure to polycyclic aromatic hydrocarbons (PAH) from yerba mate infusions in Uruguay using the margin of exposure approach (MOE) and a probabilistic method (Monte Carlo simulation). Servings/day, portion size, weekly frequency of mate consumption and body weight were the factors considered. The amount in infusions of benz[a]pyrene (B[a]P), PAH2 (sum of chrysene and B[a]P), and PAH4 (sum of benz[a]anthracene, chrysene, benz[b]fluoranthene and B[a]P) were used as markers of PAH exposure. Total content of PAH in infusions had large inter-brand variability (48-54 %) with significant differences among brands. PAH content in infusions prepared as habitually consumed was about 40 % of total content. The probability of occurrence of MOE < 10,000 varied according to the infusion preparation and the marker of exposure used, being higher for infusions prepared for total content and when B[a]P was used as marker of exposure. When the average B[a]P amount in infusion as habitually consumed was used in the simulation model, the probability of MOE < 10,000 was 9 %. The main factors contributing to B[a]P MOE variance were B[a]P amount (28.4 %), servings/day (17.3 %), and portion size (9.6 %). Heavy drinkers of yerba mate with high B[a]P content are those at risk to PAH exposure from mate infusions.
Keywords: Benz[a]pyrene; HPLC-FLD; Margin of Exposure (MOE); Monte Carlo simulation; PAH.
© 2021 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- IARC Monographs . 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
-
- EFSA Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic (Request No EFSA-Q-2004-020) EFSA J. 2005;282:1–31. doi: 10.1021/bk-2010-1048. - DOI
-
- Penning T.M. 1st ed. Humana Press; 2011. Chemical Carcinogenesis. - DOI
-
- SCF Opinion of the scientific committee on food on the risks to human health of polycyclic aromatic hydrocarbons in food. Management. 2002
LinkOut - more resources
Full Text Sources
Other Literature Sources

