Drugs for Non-alcoholic Steatohepatitis (NASH): Quest for the Holy Grail
- PMID: 33604254
- PMCID: PMC7868704
- DOI: 10.14218/JCTH.2020.00055
Drugs for Non-alcoholic Steatohepatitis (NASH): Quest for the Holy Grail
Abstract
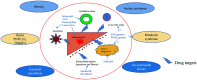
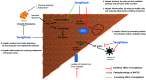
Nonalcoholic fatty liver disease (NAFLD) is a global epidemic that is likely to become the most common cause of chronic liver disease in the next decade, worldwide. Though numerous drugs have been evaluated in clinical trials, most of them have returned inconclusive results and shown poorly-tolerated adverse effects. None of the drugs have been approved by the Food and Drug Administration for treating biopsy-proven non-alcoholic steatohepatitis (NASH). Vitamin E and pioglitazone have been extensively used in treatment of biopsy-proven nondiabetic NASH patients. Although some amelioration of inflammation has been seen, these drugs did not improve the fibrosis component of NASH. Therefore, dietary modification and weight reduction have remained the cornerstone of treatment of NASH; moreover, they have shown to improve histological activity as well as fibrosis. The search for an ideal drug or 'Holy Grail' within this landscape of possible agents continues, as weight reduction is achieved only in less than 10% of patients. In this current review, we summarize the drugs for NASH which are under investigation, and we provide a critical analysis of their up-to-date results and outcomes.
Keywords: Fatty liver; NAFLD; NASH; Obeticholic acid; Saroglitazar.
© 2021 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures

References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
