Off-Label Treatments for Pediatric Psoriasis: Lessons for the Clinic
- PMID: 33604269
- PMCID: PMC7886293
- DOI: 10.2147/PTT.S268462
Off-Label Treatments for Pediatric Psoriasis: Lessons for the Clinic
Abstract
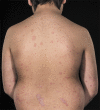
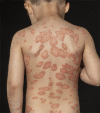
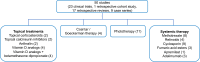
Psoriasis is a chronic inflammatory skin disease that affects up to 1.2% of children and adolescents. The treatment options for childhood psoriasis are often based on the same principles as in adults. However, most data on safety and efficacy derive from adult studies, and only a few of the frequently used treatments have achieved approval for use in children. The aim of this study was to review the current literature on off-label treatments for psoriasis in children and adolescents. We searched PubMed and identified 50 studies on off-label treatments. Of these, 23 studies were clinical trials (four randomized). There are only a small number of available studies on off-label treatments for children and adolescents with psoriasis, and many of these are retrospective reviews with few participants. Despite the current lack of studies, we still recommend the use of unapproved treatments since we have clinical experience with treatments such as topical corticosteroids, vitamin D analogs, and methotrexate that have shown promising effects. Regular clinical trials are needed to investigate the safety and efficacy of unapproved treatments. Due to The Pediatric Investigation Plans issued by The European Union, new drugs developed by pharmaceutical companies are required to undergo clinical trials in a pediatric population to get their application for marketing authorization processed. This will hopefully lead to much more data on the efficacy and safety of the new treatments, including treatments for children and adolescents with psoriasis.
Keywords: adolescents; childhood; psoriasis; unapproved treatment.
© 2021 Haulrig et al.
Conflict of interest statement
Morten B. Haulrig has received an honorarium as a consultant for Novartis. Lone Skov has been an advisor, investigator, and speaker for Abbvie, Eli Lilly, Novartis, Sanofi, Celgene, Leo pharma, BMS, UCB, and Almirall, outside the submitted work. Lone Skov reports nonfinancial support from Abbvie, Sanofi, Janssen, and grants from Novartis, Janssen, BMS, and Sanofi. Claus Zachariae has been a scientific consultant, advisor, investigator, and speaker for Eli Lilly, Jansen Cilag, Novartis, Abbvie, Takeda, Amgen, Almirall, CSL, UCB, Regeneron, MSD, and Leo Pharma.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources