Paediatric Medicines in Europe: The Paediatric Regulation-Is It Time for Reform?
- PMID: 33604345
- PMCID: PMC7884470
- DOI: 10.3389/fmed.2021.593281
Paediatric Medicines in Europe: The Paediatric Regulation-Is It Time for Reform?
Abstract
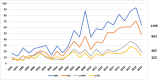
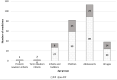
Objectives: In this paper, we investigated the effects of the European Paediatric Regulation (EC) N° 1901/2006 with respect to satisfying the paediatric therapeutic needs, assessed in terms of the increased number of paediatric medicinal products, new therapeutic indications in specific high-need conditions (neonates, oncology, rare disease, etc.) and increased number of paediatric clinical studies supporting the marketing authorisation. Methods: We analysed the paediatric medicinal products approved by the European Medicines Agency in the period January 2007-December 2019, by collecting the following data: year of approval, active substance, legal basis for the marketing authorisation, type of medicinal product (i.e., chemical, biological, or ATMP), orphan drug status, paediatric indication, Anatomical Therapeutic Chemical code (first-level), number and type of paediatric studies. Data were compared with similar data collected in the period 1996-2006. Results: In the period January 1996-December 2019, in a total of 1,190 medicinal products and 843 active substances, 34 and 38%, respectively, were paediatric. In the two periods, before and after the Paediatric Regulation implementation, the paediatric/total medicinal products ratio was constant while the paediatric/total active substances ratio decreased. Moreover, excluding generics and biosimilars, a total of 106 and 175 paediatric medicines were granted a new paediatric indication, dosage or age group in the two periods; out of 175, 128 paediatric medicines had an approved Paediatric Investigational Plan. The remaining 47 were approved without an approved Paediatric Investigational Plan, following the provisions of Directive 2001/83/EC and repurposing an off-patent drug. The analysis of the clinical studies revealed that drugs with a Paediatric Investigational Plan were supported by 3.5 studies/drug while drugs without a Paediatric Investigational Plan were supported by only 1.6 studies/drug. Discussion: This report confirms that the expectations of the European Paediatric Regulation (EC) N° 1901/2006 have been mainly satisfied. However, the reasons for the limited development of paediatric medicines in Europe, should be further discussed, taking advantage of recent initiatives in the regulatory field, such as the Action Plan on Paediatrics, and the open consultation on EU Pharmaceutical Strategy.
Keywords: EU paediatric regulation; orphan paediatric medicines; paediatric age; paediatric clinical studies; paediatric medicines; paediatric repurposing; therapeutic areas.
Copyright © 2021 Toma, Felisi, Bonifazi, Bonifazi, Giannuzzi, Reggiardo, de Wildt, Ceci and TEDDY European Network of Excellence for Paediatric Research.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- European Medicines Agency Human Medicines Highlight 2019 (2020). Available online at: https://www.ema.europa.eu/en/documents/report/human-medicines-highlights... (accessed June 24, 2020).
-
- European Parliament Council of the European Union Regulation (EC) No N° 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union; L378:1–19 (2006).
LinkOut - more resources
Full Text Sources
Other Literature Sources

