Pretreatment with S-Nitrosoglutathione Attenuates Septic Acute Kidney Injury in Rats by Inhibiting Inflammation, Oxidation, and Apoptosis
- PMID: 33604382
- PMCID: PMC7872741
- DOI: 10.1155/2021/6678165
Pretreatment with S-Nitrosoglutathione Attenuates Septic Acute Kidney Injury in Rats by Inhibiting Inflammation, Oxidation, and Apoptosis
Abstract
Objective: We aimed to investigate the protective effect of s-nitrosoglutathione (SNG) pretreatment on acute kidney injury (AKI) in septic rats.
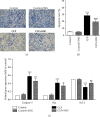
Methods: We constructed a rat model of sepsis by cecal ligation and puncture and observed the survival of the rats. We obtained kidney and blood samples from rats, observed the pathological damage to the kidney tissues, and evaluated kidney function and the expression levels of inflammatory factors. We also detected the expression of induced nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in the kidneys by immunohistochemistry and evaluated the apoptosis of kidney tubular epithelial cells (KTEC) by TUNEL.
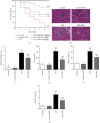
Results: Pretreatment with SNG significantly reduced the mortality of septic rats, attenuated kidney pathological damage, and decreased the levels of serum creatinine, plasma neutrophil gelatinase-associated lipocalin, and plasma kidney injury molecule-1. Moreover, SNG pretreatment decreased the levels of TNF-α and IL-1β in serum and kidney and reduced the expressions of NO, iNOS, PGE2, and COX-2 in the kidneys. Furthermore, pretreatment with SNG significantly reduced the apoptotic rate of KTEC and decreased the levels of caspase-3 and Bax mRNA, but increased the level of Bcl-2 mRNA.
Conclusion: Pretreatment with SNG has a protective effect on AKI in septic rats, and the specific mechanisms are related to inhibition of inflammation, oxidation, and apoptosis.
Copyright © 2021 Heng Fan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures
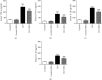

Similar articles
-
Tetrahydrocurcumin protects against sepsis-induced acute kidney injury via the SIRT1 pathway.Ren Fail. 2021 Dec;43(1):1028-1040. doi: 10.1080/0886022X.2021.1942915. Ren Fail. 2021. PMID: 34187277 Free PMC article.
-
Fish oils protects against cecal ligation and puncture‑induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis.Int J Mol Med. 2019 Nov;44(5):1771-1780. doi: 10.3892/ijmm.2019.4337. Epub 2019 Sep 11. Int J Mol Med. 2019. PMID: 31545434 Free PMC article.
-
S-nitrosoglutathione protects lipopolysaccharide-induced acute kidney injury by inhibiting toll-like receptor 4-nuclear factor-κB signal pathway.J Pharm Pharmacol. 2019 Aug;71(8):1255-1261. doi: 10.1111/jphp.13103. Epub 2019 May 22. J Pharm Pharmacol. 2019. PMID: 31115903
-
Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice.Biomed Pharmacother. 2020 Feb;122:109772. doi: 10.1016/j.biopha.2019.109772. Epub 2019 Dec 30. Biomed Pharmacother. 2020. PMID: 31918290
-
Protective Effect of Salidroside on Acute Kidney Injury in Sepsis by Inhibiting Oxidative Stress, Mitochondrial Damage, and Cell Apoptosis.Biol Pharm Bull. 2024;47(9):1550-1556. doi: 10.1248/bpb.b24-00470. Biol Pharm Bull. 2024. PMID: 39313391
Cited by
-
Clinical role of serum microRNA-155 in early diagnosis and prognosis of septic patients with acute kidney injury.Int Urol Nephrol. 2024 May;56(5):1687-1694. doi: 10.1007/s11255-023-03855-z. Epub 2023 Oct 28. Int Urol Nephrol. 2024. PMID: 37898565
-
Xiaoyu Xiezhuo Drink Protects against Ischemia-Reperfusion Acute Kidney Injury in Aged Mice through Inhibiting the TGF-β1/Smad3 and HIF1 Signaling Pathways.Biomed Res Int. 2021 Sep 9;2021:9963732. doi: 10.1155/2021/9963732. eCollection 2021. Biomed Res Int. 2021. PMID: 34545331 Free PMC article.
-
New findings on the antagonism of the environmental chemical toxicity 2-ethylhexyl diphenyl phosphate: Glycyrrhizic acid as an Nrf2 activator targets Nrf2/ROS/STAT3 signalling crosstalk to alleviate thymic injury in chicks.Poult Sci. 2025 Apr;104(4):104918. doi: 10.1016/j.psj.2025.104918. Epub 2025 Feb 25. Poult Sci. 2025. PMID: 40024011 Free PMC article.
-
No safe renal warm ischemia time-The molecular network characteristics and pathological features of mild to severe ischemia reperfusion kidney injury.Front Mol Biosci. 2022 Nov 16;9:1006917. doi: 10.3389/fmolb.2022.1006917. eCollection 2022. Front Mol Biosci. 2022. PMID: 36465563 Free PMC article.
-
Protective effect of N-acetylcysteine on acute lung injury in septic rats by inhibiting inflammation, oxidation, and apoptosis.Iran J Basic Med Sci. 2022 Jul;25(7):859-864. doi: 10.22038/IJBMS.2022.65350.14384. Iran J Basic Med Sci. 2022. PMID: 36033949 Free PMC article.
References
-
- Mehta R. L., Program to Improve Care in Acute Renal Disease (PICARD) Study Group, Bouchard J., et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Medicine. 2011;37(2):241–248. doi: 10.1007/s00134-010-2089-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

