Self-Collected Oral Fluid Saliva Is Insensitive Compared With Nasal-Oropharyngeal Swabs in the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in Outpatients
- PMID: 33604399
- PMCID: PMC7798743
- DOI: 10.1093/ofid/ofaa648
Self-Collected Oral Fluid Saliva Is Insensitive Compared With Nasal-Oropharyngeal Swabs in the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in Outpatients
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic control will require widespread access to accurate diagnostics. Salivary sampling circumvents swab supply chain bottlenecks, is amenable to self-collection, and is less likely to create an aerosol during collection compared with the nasopharyngeal swab.
Methods: We compared real-time reverse-transcription polymerase chain reaction Abbott m2000 results from matched salivary oral fluid (gingival crevicular fluid collected in an Oracol device) and nasal-oropharyngeal (OP) self-collected specimens in viral transport media from a nonhospitalized, ambulatory cohort of coronavirus disease 2019 (COVID-19) patients at multiple time points. These 2 sentences should be at the beginning of the results.
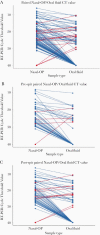
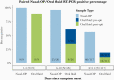
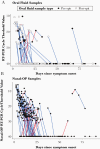
Results: There were 171 matched specimen pairs. Compared with nasal-OP swabs, 41.6% of the oral fluid samples were positive. Adding spit to the oral fluid percent collection device increased the percent positive agreement from 37.2% (16 of 43) to 44.6% (29 of 65). The positive percent agreement was highest in the first 5 days after symptoms and decreased thereafter. All of the infectious nasal-OP samples (culture positive on VeroE6 TMPRSS2 cells) had a matched SARS-CoV-2 positive oral fluid sample.
Conclusions: In this study of nonhospitalized SARS-CoV-2-infected persons, we demonstrate lower diagnostic sensitivity of self-collected oral fluid compared with nasal-OP specimens, a difference that was especially prominent more than 5 days from symptom onset. These data do not justify the routine use of oral fluid collection for diagnosis of SARS-CoV-2 despite the greater ease of collection. It also underscores the importance of considering the method of saliva specimen collection and the time from symptom onset especially in outpatient populations.
Keywords: COVID-19; SARS-CoV-2; coronavirus; outpatient; saliva.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

