Milk modulates macrophage polarization in vitro
- PMID: 33604549
- PMCID: PMC7885867
- DOI: 10.1016/j.cytox.2019.100009
Milk modulates macrophage polarization in vitro
Abstract
Objective: Milk holds an anti-inflammatory response that is particularly important to protecting infants against necrotizing enterocolitis. Milk might also exert anti-inflammatory effects in adulthood, including the oral cavity where macrophages of the oral mucosal control innate immunity defense. It remains unknown, however, whether milk can modulate the local inflammatory response by affecting the polarization of macrophages.
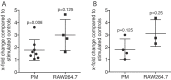
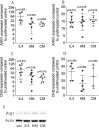
Material and methods: To determine whether pasteurized human milk and pasteurized cow milk can provoke macrophage polarization, murine bone marrow macrophages and RAW264.7 cells were exposed to human saliva or the inflammatory cytokines IL1β and TNFα. Activation of pro-(M1) inflammatory response is indicated by the expression of IL1 and IL8. To determine polarization towards a M2 phenotype, the expression of arginase 1 (ARG1) and chitinase-like 3 (Chil3) was determined by reverse transcriptase PCR and immunoassay. Western blot was done on phosphorylated p38 and JNK.
Results: Aqueous fractions of human milk and cow milk from different donors, respectively, significantly decreased the inflammatory response of primary macrophages and RAW264.7 cells when exposed to saliva or IL1 and TNFα. Similar to IL4, human milk and cow milk caused a robust expression of ARG1 and Chil3 in primary macrophages. The polarization of macrophages by pasteurized milk occurred independent of the phosphorylation of p38 and JNK.
Conclusion: These data suggest that pasteurized milk, independent of the origin, can cause the polarization of macrophages from a pro-inflammatory M1 towards a pro-resolving M2 phenotype. Thus, milk might have a protective role for the oral cavity by modulation of the macrophage-based innate immune system.
Keywords: Inflammation; Macrophages; Milk; Mucositis; Oral; Polarisation.
© 2019 The Author(s).
Figures




Similar articles
-
The anti-inflammatory effect of milk and dairy products on periodontal cells: an in vitro approach.Clin Oral Investig. 2019 Apr;23(4):1959-1966. doi: 10.1007/s00784-018-2642-4. Epub 2018 Sep 20. Clin Oral Investig. 2019. PMID: 30238412
-
Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro.J Periodontol. 2020 Feb;91(2):244-252. doi: 10.1002/JPER.19-0216. Epub 2019 Sep 14. J Periodontol. 2020. PMID: 31376159 Free PMC article.
-
Saliva initiates the formation of pro-inflammatory macrophages in vitro.Arch Oral Biol. 2017 Jan;73:295-301. doi: 10.1016/j.archoralbio.2016.10.012. Epub 2016 Oct 20. Arch Oral Biol. 2017. PMID: 27825074
-
Macrophages: The Good, the Bad, and the Gluttony.Front Immunol. 2021 Aug 12;12:708186. doi: 10.3389/fimmu.2021.708186. eCollection 2021. Front Immunol. 2021. PMID: 34456917 Free PMC article. Review.
-
MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response.Shock. 2016 Aug;46(2):122-31. doi: 10.1097/SHK.0000000000000604. Shock. 2016. PMID: 26954942 Free PMC article. Review.
Cited by
-
RNAseq of TGF-β receptor type I kinase-dependent genes in oral fibroblast exposed to milk.BMC Oral Health. 2021 Nov 16;21(1):581. doi: 10.1186/s12903-021-01913-5. BMC Oral Health. 2021. PMID: 34789212 Free PMC article.
-
Evaluation of the Association between Amount and Type of Milk Consumption and Periodontitis: Data from the Korea National Health and Nutrition Examination Survey (2016-2018).Nutrients. 2023 Feb 11;15(4):914. doi: 10.3390/nu15040914. Nutrients. 2023. PMID: 36839272 Free PMC article.
-
Damaged Mesenchymal Cells Dampen the Inflammatory Response of Macrophages and the Formation of Osteoclasts.J Clin Med. 2022 Jul 14;11(14):4061. doi: 10.3390/jcm11144061. J Clin Med. 2022. PMID: 35887825 Free PMC article.
-
Flavonoids from Dalbergia cochinchinensis: Impact on osteoclastogenesis.J Dent Sci. 2023 Jan;18(1):112-119. doi: 10.1016/j.jds.2022.06.026. Epub 2022 Jul 15. J Dent Sci. 2023. PMID: 36643234 Free PMC article.
-
Prevention of Chronic Morbidities in Extremely Premature Newborns with LISA-nCPAP Respiratory Therapy and Adjuvant Perinatal Strategies.Antioxidants (Basel). 2023 May 24;12(6):1149. doi: 10.3390/antiox12061149. Antioxidants (Basel). 2023. PMID: 37371878 Free PMC article. Review.
References
-
- Delima A.J., Van Dyke T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontology. 2003;2000(31):55–76. - PubMed
-
- Darveau R.P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8(7):481–490. - PubMed
-
- Berglundh T., Zitzmann N.U., Donati M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011;38(Suppl 11):188–202. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

