Human osteoclasts/osteoblasts 3D dynamic co‑culture system to study the beneficial effects of glucosamine on bone microenvironment
- PMID: 33604678
- PMCID: PMC7910015
- DOI: 10.3892/ijmm.2021.4890
Human osteoclasts/osteoblasts 3D dynamic co‑culture system to study the beneficial effects of glucosamine on bone microenvironment
Abstract
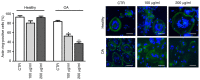
Glucosamine (GlcN) functions as a building block of the cartilage matrix, and its multifaceted roles in promoting joint health have been extensively investigated. However, the role of GlcN in osteogenesis and bone tissue is poorly understood, mainly due to the lack of adequate experimental models. As a result, the benefit of GlcN application in bone disorders remains controversial. In order to further elucidate the pharmacological relevance and potential therapeutic/nutraceutic efficacy of GlcN, the effect of GlcN treatment was investigated in human primary osteoclasts (hOCs) and osteoblasts (hOBs) that were cultured with two‑dimensional (2D) traditional methods or co‑cultured in a 3D dynamic system more closely resembling the in vivo bone microenvironment. Under these conditions, osteoclastogenesis was supported by hOBs and sizeable self‑assembling aggregates were obtained. The differentiated hOCs were evaluated using tartrate‑resistant acid phosphatase assays and osteogenic differentiation was monitored by analyzing mineral matrix deposition via Alizarin Red staining, with expression of specific osteogenic markers determined via reverse transcription‑quantitative PCR. It was found that crystalline GlcN sulfate was effective in decreasing osteoclastic cell differentiation and function. hOCs isolated from patients with OA were more sensitive compared with those from healthy donors. Additionally, GlcN exhibited anabolic effects on hOCs both in 2D conventional cell culture and in hOC/hOB 3D dynamic co‑culture. The present study demonstrated for the first time the effectiveness of a 3D dynamic co‑culture system for characterizing the spectrum of action of GlcN on the bone microenvironment, which may pave the way for more fully determining the potential applications of a compound such as GlcN, which is positioned between pharmaceuticals and nutraceuticals. Based on the present findings, it is hypothesized that GlcN may have potential benefits in the treatment of osteopenic diseases such as osteoporosis, as well as in bone maintenance.
Keywords: glucosamine; bone tissue; bone cells; 3D culture system; osteoarthritis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Effect of glucosamine, a therapeutic agent for osteoarthritis, on osteoblastic cell differentiation.Int J Mol Med. 2011 Sep;28(3):373-9. doi: 10.3892/ijmm.2011.686. Epub 2011 Apr 29. Int J Mol Med. 2011. PMID: 21537831
-
Establishment of a 3D-dynamic osteoblasts-osteoclasts co-culture model to simulate the jawbone microenvironment in vitro.Life Sci. 2016 May 1;152:82-93. doi: 10.1016/j.lfs.2016.03.035. Epub 2016 Mar 22. Life Sci. 2016. PMID: 27015789
-
Biological activities of glucosamine and its related substances.Adv Food Nutr Res. 2012;65:337-52. doi: 10.1016/B978-0-12-416003-3.00022-6. Adv Food Nutr Res. 2012. PMID: 22361198 Review.
-
Glucosamines Attenuate Bone Loss Due to Menopause by Regulating Osteoclast Function in Ovariectomized Mice.Biol Pharm Bull. 2016;39(6):1035-41. doi: 10.1248/bpb.b16-00066. Biol Pharm Bull. 2016. PMID: 27251507
-
From the Clinical Problem to the Basic Research-Co-Culture Models of Osteoblasts and Osteoclasts.Int J Mol Sci. 2018 Aug 3;19(8):2284. doi: 10.3390/ijms19082284. Int J Mol Sci. 2018. PMID: 30081523 Free PMC article. Review.
Cited by
-
The Influence of Vitamin C-incorporated Polycaprolactone on Osteogenesis in Osteoblast-Osteoclast Co-culture In Vitro.Int Dent J. 2025 Aug 20;75(5):100949. doi: 10.1016/j.identj.2025.100949. Online ahead of print. Int Dent J. 2025. PMID: 40840307 Free PMC article.
-
Adipose-Derived Stromal Cell Conditioned Medium on Bone Remodeling: Insights from a 3D Osteoblast-Osteoclast Co-Culture Model.Calcif Tissue Int. 2025 Jan 7;116(1):26. doi: 10.1007/s00223-024-01335-9. Calcif Tissue Int. 2025. PMID: 39774716
-
Three-Dimensional Co-Culture System of Human Osteoblasts and Osteoclast Precursors from Osteoporotic Patients as an Innovative Model to Study the Role of Nutrients: Focus on Vitamin K2.Nutrients. 2021 Aug 17;13(8):2823. doi: 10.3390/nu13082823. Nutrients. 2021. PMID: 34444982 Free PMC article.
-
Advances in biomaterial-based composite spheroid for articular cartilage regeneration.J Tissue Eng. 2025 Jul 1;16:20417314251349669. doi: 10.1177/20417314251349669. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 40626181 Free PMC article. Review.
-
Effect of Chitosan Degradation Products, Glucosamine and Chitosan Oligosaccharide, on Osteoclastic Differentiation.BioTech (Basel). 2024 Mar 6;13(1):6. doi: 10.3390/biotech13010006. BioTech (Basel). 2024. PMID: 38534915 Free PMC article.
References
-
- Agiba AM. Nutraceutical formulations containing glucosamine and chondroitin sulphate in the treatment of osteoarthritis: Emphasis on clinical efficacy and formulation challenges. Int J Curr Pharm Res. 2017;9:1–7. doi: 10.22159/ijcpr.2017v9i2.17380. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

