High-throughput detection of antibodies targeting the SARS-CoV-2 Spike in longitudinal convalescent plasma samples
- PMID: 33604922
- PMCID: PMC8013554
- DOI: 10.1111/trf.16318
High-throughput detection of antibodies targeting the SARS-CoV-2 Spike in longitudinal convalescent plasma samples
Abstract
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is the cause of the ongoing coronavirus disease 2019 (COVID-19) pandemic, infecting millions of people and causing more than two million deaths. The SARS-CoV-2 Spike glycoproteins mediate viral entry and represent the main target for antibody responses. Humoral responses were shown to be important for preventing and controlling infection by coronaviruses. A promising approach to reduce the severity of COVID-19 is the transfusion of convalescent plasma. However, longitudinal studies revealed that the level of antibodies targeting the receptor-binding domain (RBD) of the SARS-CoV-2 Spike declines rapidly after the resolution of the infection.
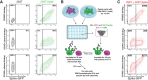
Study design and methods: To extend this observation beyond the RBD domain, we performed a longitudinal analysis of the persistence of antibodies targeting the full-length SARS-CoV-2 Spike in the plasma from 15 convalescent donors. We generated a 293T cell line constitutively expressing the SARS-CoV-2 Spike and used it to develop a high-throughput flow cytometry-based assay to detect SARS-CoV-2 Spike-specific antibodies in the plasma of convalescent donors.
Results and conclusion: We found that the level of antibodies targeting the full-length SARS-CoV-2 Spike declines gradually after the resolution of the infection. This decline was not related to the number of donations but strongly correlated with the decline of RBD-specific antibodies and the number of days post-symptom onset. These findings help to better understand the decline of humoral responses against the SARS-CoV-2 Spike and provide important information on when to collect plasma after recovery from active infection for convalescent plasma transfusion.
Keywords: COVID-19; RBD; SARS-CoV-2; antibodies; convalescent plasma; coronavirus; flow cytometry; high-throughput screening; spike glycoproteins.
© 2021 AABB.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

