The Luteinizing Hormone Receptor Knockout Mouse as a Tool to Probe the In Vivo Actions of Gonadotropic Hormones/Receptors in Females
- PMID: 33605422
- PMCID: PMC8171189
- DOI: 10.1210/endocr/bqab035
The Luteinizing Hormone Receptor Knockout Mouse as a Tool to Probe the In Vivo Actions of Gonadotropic Hormones/Receptors in Females
Abstract
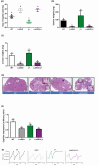
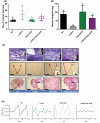
Mouse models with altered gonadotropin functions have provided invaluable insight into the functions of these hormones/receptors. Here we describe the repurposing of the infertile and hypogonadal luteinizing hormone receptor (LHR) knockout mouse model (LuRKO), to address outstanding questions in reproductive physiology. Using crossbreeding strategies and physiological and histological analyses, we first addressed the physiological relevance of forced LHR homomerization in female mice using BAC expression of 2 ligand-binding and signaling deficient mutant LHR, respectively, that have previously shown to undergo functional complementation and rescue the hypogonadal phenotype of male LuRKO mice. In female LuRKO mice, coexpression of signaling and binding deficient LHR mutants failed to rescue the hypogonadal and anovulatory phenotype. This was apparently due to the low-level expression of the 2 mutant LHR and potential lack of luteinizing hormone (LH)/LHR-dependent pleiotropic signaling that has previously been shown at high receptor densities to be essential for ovulation. Next, we utilized a mouse model overexpressing human chorionic gonadotropin (hCG) with increased circulating "LH/hCG"-like bioactivity to ~40 fold higher than WT females, to determine if high circulating hCG in the LuRKO background could reveal putative LHR-independent actions. No effects were found, thus, suggesting that LH/hCG mediate their gonadal and non-gonadal effects solely via LHR. Finally, targeted expression of a constitutively active follicle stimulating hormone receptor (FSHR) progressed antral follicles to preovulatory follicles and displayed phenotypic markers of enhanced estrogenic activity but failed to induce ovulation in LuRKO mice. This study highlights the critical importance and precise control of functional LHR and FSHR for mediating ovarian functions and of the potential repurposing of existing genetically modified mouse models in answering outstanding questions in reproductive physiology.
Keywords: G protein-coupled receptors; follicle-stimulating hormone; gonadotropin hormones; luteinizing hormone; reproduction.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Similar articles
-
Specificity of cognate ligand-receptor interactions: fusion proteins of human chorionic gonadotropin and the heptahelical receptors for human luteinizing hormone, thyroid-stimulating hormone, and follicle-stimulating hormone.Endocrinology. 2003 Jan;144(1):129-37. doi: 10.1210/en.2002-220829. Endocrinology. 2003. PMID: 12488338
-
Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice.Mol Endocrinol. 2001 Jan;15(1):172-83. doi: 10.1210/mend.15.1.0582. Mol Endocrinol. 2001. PMID: 11145748
-
Functional characterization of follicle-stimulating hormone and luteinizing hormone receptors in cloudy catshark, Scyliorhinus torazame.Gen Comp Endocrinol. 2024 Aug 1;354:114542. doi: 10.1016/j.ygcen.2024.114542. Epub 2024 Apr 27. Gen Comp Endocrinol. 2024. PMID: 38685391
-
Gonadotropins and their receptors: coevolution, genetic variants, receptor imaging, and functional antagonists.Biol Reprod. 2018 Jul 1;99(1):3-12. doi: 10.1093/biolre/ioy012. Biol Reprod. 2018. PMID: 29462242 Review.
-
The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited.Int J Mol Sci. 2021 Nov 25;22(23):12735. doi: 10.3390/ijms222312735. Int J Mol Sci. 2021. PMID: 34884539 Free PMC article. Review.
Cited by
-
Pharmacogenomic of LH and its receptor: are we ready for clinical practice?Reprod Biol Endocrinol. 2025 Feb 25;23(Suppl 1):29. doi: 10.1186/s12958-025-01359-2. Reprod Biol Endocrinol. 2025. PMID: 40001128 Free PMC article. Review.
References
-
- Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179(1-2):39-46. - PubMed
-
- Jonas KC, Oduwole OO, Peltoketo H, Rulli SB, Huhtaniemi IT. Mouse models of altered gonadotrophin action: insight into male reproductive disorders. Reproduction. 2014;148(4):R63-R70. - PubMed
-
- Ulloa-Aguirre A, Reiter E, Bousfield G, Dias JA, Huhtaniemi I. Constitutive activity in gonadotropin receptors. Adv Pharmacol. 2014;70:37-80. - PubMed
-
- Tao YX, Segaloff DL. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog Mol Biol Transl Sci. 2009;89:115-131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

