Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial
- PMID: 33605656
- PMCID: PMC8357842
- DOI: 10.1097/j.pain.0000000000002234
Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial
Abstract
It is commonly believed that blinding to treatment assignment is necessary for placebos to have an effect. However, placebos administered without concealment (ie, open-label placebos [OLPs]) have recently been shown to be effective in some conditions. This study had 2 objectives: first, to determine whether OLP treatment is superior to no-pill control (NPC) in irritable bowel syndrome (IBS) and, second, to compare the efficacy of OLP against double-blind placebo (DBP). In a 6-week, 3-arm, randomized clinical trial, participants were randomized in equal proportions to 3 arms: OLP, DBP, or NPC. Two hundred sixty-two adults (72.9% women), with a mean age of 42.0 (SD = 18.1) years, participated in the primary study. The mean improvement on the IBS Severity Scoring System from baseline to the 6-week end point was significantly greater in OLP compared with that in NPC (90.6 vs 52.3, P = 0.038). Open-label placebo and DBP did not differ significantly on IBS Severity Scoring System improvement (100.3 vs 90.6, P = 0.485). Standardized effect sizes were moderate for OLP vs NPC (d = 0.43) and small for OLP vs DBP (d = 0.10). Participants treated with OLP reported clinically meaningful improvements in IBS symptoms that were significantly greater than those on NPC. Open-label placebo and DBP had similar effects that did not differ significantly, suggesting that blinding may not be necessary for placebos to be effective and that OLP could play a role in the management of patients with refractory IBS.
Trial registration: ClinicalTrials.gov NCT02802241.
Copyright © 2021 International Association for the Study of Pain.
Figures

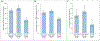
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

