Exploring the evidence behind the comparable impact of the pneumococcal conjugate vaccines PHiD-CV and PCV13 on overall pneumococcal disease
- PMID: 33605846
- PMCID: PMC8920200
- DOI: 10.1080/21645515.2021.1872341
Exploring the evidence behind the comparable impact of the pneumococcal conjugate vaccines PHiD-CV and PCV13 on overall pneumococcal disease
Abstract
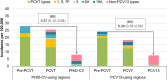
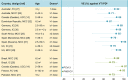
The worldwide implementation of pneumococcal conjugate vaccines (PCVs) in children has reduced the overall pneumococcal disease burden. Two PCVs are widely available for infant vaccination: the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) and the 13-valent PCV (PCV13). While these PCVs differ in serotype composition (PCV13 includes polysaccharides of serotypes 3, 6A and 19A; PHiD-CV does not), their impact on the overall pneumococcal disease burden in children is comparable. This commentary summarizes the evidence of comparability between PHiD-CV and PCV13 and explores why differences in serotype composition may not necessarily translate into a differential clinical impact. Both vaccines confer similarly high protection against disease caused by vaccine serotypes and lead to a partial replacement by non-vaccine serotypes. PHiD-CV does not protect against serotype 3 disease (not included in the vaccine) and PCV13's effect on this serotype has been inconsistent. PHiD-CV provides some cross-protection against disease caused by vaccine-related serotype 19A but neither vaccine has fully controlled 19A disease. While protection against 19A is higher for PCV13 than PHiD-CV, replacement by non-PCV13 serotypes in settings with a PCV13 program appears to compensate for this difference. This results in a similar residual overall disease burden with both vaccines.
Keywords: PCV10; PCV13; PHiD-CV; Pneumococcal conjugate vaccine; children; invasive pneumococcal disease; otitis media; pneumonia; replacement; serotypes.
Plain language summary
PLAIN LANGUAGE SUMMARYWhat is the context?The pneumococcus bacterium can cause infections of the meninges, blood, lung, middle ear and sinuses.Two vaccins, Synflorix (GSK) and Prevnar 13 (Pfizer Inc.), are widely used to protect young children against these infections.The vaccines’ compositions differ: Synflorix includes antigens from 10 pneumococcus strains (or “serotypes”) and Prevnar 13 from 13 serotypes.However, both have a similar effect on the total pneumococcal disease burden in children.What does this commentary highlight?This commentary summarizes the evidence beihnd the two vaccines’ comparable impact on pneumococcal disase.It also looks at why the vaccines have a similar effect on the total pneumococcal disease burden despite their different compositions.What is the impact on current thinking?Given that Synflorix and Prevnar 13 have a comparable impact on pneumococcal disease, a country’s choice between the two vaccines will depend on vaccine supply, cost, logistical factors (e.g., transport, storage, training requirements of health workers) and the local pneumococcal epidemiology.
Figures


References
-
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Luksic I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e757. doi: 10.1016/s2214-109x(18)30247-x. - DOI - PMC - PubMed
-
- World Health Organization . Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec. 2019;94(8):85–104.
-
- Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, Gladstone RA, Turner P, Keenan JD, Breiman RF, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020;11(3):e00937–00920. doi: 10.1128/mBio.00937-20. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
