Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials
- PMID: 33606033
- PMCID: PMC7896193
- DOI: 10.1001/jamanetworkopen.2020.37640
Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials
Abstract
Importance: Medical research has not equitably included members of racial/ethnic minority groups or female and older individuals. There are limited data on participant demographic characteristics in vaccine trials despite the importance of these data to current trials aimed at preventing coronavirus disease 2019.
Objective: To investigate whether racial/ethnic minority groups and female and older adults are underrepresented among participants in vaccine clinical trials.
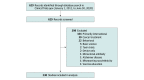
Design, setting, and participants: This cross-sectional study examined data from completed US-based vaccine trials registered on ClinicalTrials.gov from July 1, 2011, through June 30, 2020. The terms vaccine, vaccination, immunization, and inoculation were used to identify trials. Only those addressing vaccine immunogenicity or efficacy of preventative vaccines were included.
Main outcomes and measures: The numbers and percentages of racial/ethnic minority, female, and older individuals compared with US census data from 2011 and 2018. Secondary outcome measures were inclusion by trial phase and year of completion.
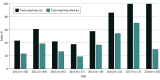
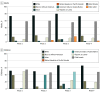
Results: A total of 230 US-based trials with 219 555 participants were included in the study. Most trials were randomized (180 [78.3%]), included viral vaccinations (159 [69.1%]), and represented all trial phases. Every trial reported age and sex; 134 (58.3%) reported race and 79 (34.3%) reported ethnicity. Overall, among adult study participants, White individuals were overrepresented (77.9%; 95% CI, 77.4%-78.4%), and Black or African American individuals (10.6%; 95% CI, 10.2%-11.0%) and American Indian or Alaska Native individuals (0.4%; 95% CI, 0.3%-0.5%) were underrepresented compared with US census data; enrollment of Asian individuals was similar (5.7%; 95% CI, 5.5%-6.0%). Enrollment of Hispanic or Latino individuals (11.6%; 95% CI, 11.1%-12.0%) was also low even among the limited number of adult trials reporting ethnicity. Adult trials were composed of more female participants (75 325 [56.0%]), but among those reporting age as a percentage, enrollment of participants who were aged 65 years or older was low (12.1%; 95% CI, 12.0%-12.3%). Black or African American participants (10.1%; 95% CI, 9.7%-10.6%) and Hispanic or Latino participants (22.5%; 95% CI, 21.6%-23.4%) were also underrepresented in pediatric trials. Among trials reporting race/ethnicity, 65 (48.5%) did not include American Indian or Alaska Native participants and 81 (60.4%) did not include Hawaiian or Pacific Islander participants.
Conclusions and relevance: This cross-sectional study found that among US-based vaccine clinical trials, members of racial/ethnic minority groups and older adults were underrepresented, whereas female adults were overrepresented. These findings suggest that diversity enrollment targets should be included for all vaccine trials targeting epidemiologically important infections.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical