Development of nasal allergen challenge with cockroach in children with asthma
- PMID: 33606312
- PMCID: PMC8503840
- DOI: 10.1111/pai.13480
Development of nasal allergen challenge with cockroach in children with asthma
Abstract
Background: Nasal allergen challenge (NAC) could be a means to assess indication and/or an outcome of allergen-specific therapies, particularly for perennial allergens. NACs are not commonly conducted in children with asthma, and cockroach NACs are not well established. This study's objective was to identify a range of German cockroach extract doses that induce nasal symptoms and to assess the safety of cockroach NAC in children with asthma.
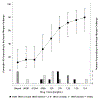
Methods: Ten adults (18-37 years) followed by 25 children (8-14 years) with well-controlled, persistent asthma and cockroach sensitization underwent NAC with diluent followed by up to 8 escalating doses of cockroach extract (0.00381-11.9 µg/mL Bla g 1). NAC outcome was determined by Total Nasal Symptom Score (TNSS) and/or sneeze score. Cockroach allergen-induced T-cell activation and IL-5 production were measured in peripheral blood mononuclear cells.
Results: 67% (6/9) of adults and 68% (17/25) of children had a positive NAC at a median response dose of 0.120 µg/mL [IQR 0.0380-0.379 µg/mL] of Bla g 1. Additionally, three children responded to diluent alone and did not receive any cockroach extract. Overall, 32% (11/34) were positive with sneezes alone, 15% (5/34) with TNSS alone, and 21% (7/34) with both criteria. At baseline, NAC responders had higher cockroach-specific IgE (P = .03), lower cockroach-specific IgG/IgE ratios (children, P = .002), and increased cockroach-specific IL-5-producing T lymphocytes (P = .045). The NAC was well tolerated.
Conclusion: We report the methodology of NAC development for children with persistent asthma and cockroach sensitization. This NAC could be considered a tool to confirm clinically relevant sensitization and to assess responses in therapeutic studies.
Trial registration: ClinicalTrials.gov NCT02710136.
Keywords: allergic rhinitis; asthma; children; cockroach allergy; inner city; nasal allergen challenge.
© 2021 European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Figures


References
-
- Togias A, Corren J, Wagenmann M. Nasal Provocation Testing. In: Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons ER, Lemanske RFJ, eds. Middleton’s Textbook of Allergy. 7th ed: Elsevier; 2009:1281–94.
-
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997;336(19):1356–63. - PubMed
-
- Sastre J, Ibanez MD, Lombardero M, Laso MT, Lehrer S. Allergy to cockroaches in patients with asthma and rhinitis in an urban area (Madrid). Allergy 1996; 51: 582–586. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- HHSN272200900052C/AI/NIAID NIH HHS/United States
- HHSN272201000052I/AI/NIAID NIH HHS/United States
- UL1 TR000451/TR/NCATS NIH HHS/United States
- UM1 AI114271/AI/NIAID NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

