Dysregulation of the centrosome induced by BRCA1 deficiency contributes to tissue-specific carcinogenesis
- PMID: 33606355
- PMCID: PMC8088922
- DOI: 10.1111/cas.14859
Dysregulation of the centrosome induced by BRCA1 deficiency contributes to tissue-specific carcinogenesis
Abstract
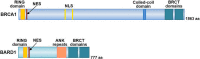
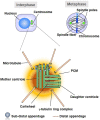
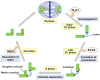
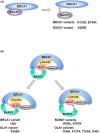
Alterations in breast cancer gene 1 (BRCA1), a tumor suppressor gene, increase the risk of breast and ovarian cancers. BRCA1 forms a heterodimer with BRCA1-associated RING domain protein 1 (BARD1) and functions in multiple cellular processes, including DNA repair and centrosome regulation. BRCA1 acts as a tumor suppressor by promoting homologous recombination (HR) repair, and alterations in BRCA1 cause HR deficiency, not only in breast and ovarian tissues but also in other tissues. The molecular mechanisms underlying BRCA1 alteration-induced carcinogenesis remain unclear. Centrosomes are the major microtubule-organizing centers and function in bipolar spindle formation. The regulation of centrosome number is critical for chromosome segregation in mitosis, which maintains genomic stability. BRCA1/BARD1 function in centrosome regulation together with Obg-like ATPase (OLA1) and receptor for activating protein C kinase 1 (RACK1). Cancer-derived variants of BRCA1, BARD1, OLA1, and RACK1 do not interact, and aberrant expression of these proteins results in abnormal centrosome duplication in mammary-derived cells, and rarely in other cell types. RACK1 is involved in centriole duplication in the S phase by promoting polo-like kinase 1 activation by Aurora A, which is critical for centrosome duplication. Centriole number is higher in cells derived from mammary tissues compared with in those derived from other tissues, suggesting that tissue-specific centrosome characterization may shed light on the tissue specificity of BRCA1-associated carcinogenesis. Here, we explored the role of the BRCA1-containing complex in centrosome regulation and the effect of its deficiency on tissue-specific carcinogenesis.
Keywords: BRCA1; HBOC; breast cancer; centrosome; centrosome amplification.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest for this article.
Figures
Similar articles
-
The Function of BARD1 in Centrosome Regulation in Cooperation with BRCA1/OLA1/RACK1.Genes (Basel). 2020 Jul 24;11(8):842. doi: 10.3390/genes11080842. Genes (Basel). 2020. PMID: 32722046 Free PMC article. Review.
-
RACK1 regulates centriole duplication by controlling localization of BRCA1 to the centrosome in mammary tissue-derived cells.Oncogene. 2019 Apr;38(16):3077-3092. doi: 10.1038/s41388-018-0647-8. Epub 2019 Jan 7. Oncogene. 2019. PMID: 30617304
-
The BRCA1/BARD1-interacting protein OLA1 functions in centrosome regulation.Mol Cell. 2014 Jan 9;53(1):101-14. doi: 10.1016/j.molcel.2013.10.028. Epub 2013 Nov 27. Mol Cell. 2014. PMID: 24289923
-
Roles of RACK1 in centrosome regulation and carcinogenesis.Cell Signal. 2022 Feb;90:110207. doi: 10.1016/j.cellsig.2021.110207. Epub 2021 Nov 27. Cell Signal. 2022. PMID: 34843916 Review.
-
BRCA1-Interacting Protein OLA1 Requires Interaction with BARD1 to Regulate Centrosome Number.Mol Cancer Res. 2018 Oct;16(10):1499-1511. doi: 10.1158/1541-7786.MCR-18-0269. Epub 2018 Jun 1. Mol Cancer Res. 2018. PMID: 29858377
Cited by
-
Identification of a centrosome-related prognostic signature for breast cancer.Front Oncol. 2023 Mar 22;13:1138049. doi: 10.3389/fonc.2023.1138049. eCollection 2023. Front Oncol. 2023. PMID: 37035151 Free PMC article.
-
Comprehensive Analysis of the Significance of Breast Cancer Gene 1 (BRCA-1) in Bladder Cancer.Cancer Manag Res. 2024 Sep 30;16:1305-1319. doi: 10.2147/CMAR.S467817. eCollection 2024. Cancer Manag Res. 2024. PMID: 39372705 Free PMC article.
-
Moonlighting at the Poles: Non-Canonical Functions of Centrosomes.Front Cell Dev Biol. 2022 Jul 14;10:930355. doi: 10.3389/fcell.2022.930355. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912107 Free PMC article. Review.
-
BRCA1 transports the DNA damage signal for CDDP-induced centrosome amplification through the centrosomal Aurora A.Cancer Sci. 2022 Dec;113(12):4230-4243. doi: 10.1111/cas.15573. Epub 2022 Sep 21. Cancer Sci. 2022. PMID: 36082621 Free PMC article.
-
Assessment of small in-frame indels and C-terminal nonsense variants of BRCA1 using a validated functional assay.Sci Rep. 2022 Sep 28;12(1):16203. doi: 10.1038/s41598-022-20500-4. Sci Rep. 2022. PMID: 36171434 Free PMC article.
References
-
- Miki Y, Swensen J, Shattuck‐Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science (80). 1994;266:66‐71. - PubMed
-
- Dine J, Deng C‐X. Mouse models of BRCA1 and their application to breast cancer research. Cancer Metastasis Rev. 2013;32:25‐37. - PubMed
-
- Takaoka M, Miki Y. BRCA1 gene: function and deficiency. Int J Clin Oncol. 2018;23:36‐44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

