Ultrasound-Targeted Microbubble Destruction Enhances the Inhibitive Efficacy of miR-21 Silencing in HeLa Cells
- PMID: 33606670
- PMCID: PMC7901158
- DOI: 10.12659/MSM.923660
Ultrasound-Targeted Microbubble Destruction Enhances the Inhibitive Efficacy of miR-21 Silencing in HeLa Cells
Abstract
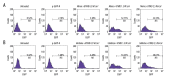
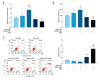
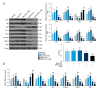
BACKGROUND Previous studies have shown that miR-21 upregulation is related to the aggressive development of cervical cancer. Ultrasound-targeted microbubble destruction (UTMD) is a method that increases the absorption of targeted genes or drugs by cells. We focus on the role of UTMD-mediated miR-21 transfection in HeLa cells, a cervical cancer cell line. MATERIAL AND METHODS The effects of different ultrasound intensities on the transfection efficiency of miR-21-enhanced green fluorescent protein (EGFP) and miR-21 inhibitor-EGFP plasmids were determined by flow cytometry. The effects of UTMD-mediated miR-21 transfection on HeLa cell proliferation, apoptosis, migration, and invasion were measured by CCK-8, flow cytometry, wound healing experiments, and transwell migration assay, respectively. Western blot and real-time quantitative PCR were used to detect the expression of tumor-related genes. RESULTS When the ultrasound intensity was 1.5 W/cm², the miR-21 plasmid had the highest transfection efficiency. Exogenous miR-21 promoted cell proliferation, migration, and invasion, and inhibited cell apoptosis in HeLa cells. Treatment of cells with UTMD further enhanced the effects of miR-21-EGFP and miR-21 inhibitor-EGFP. In addition, miR-21 overexpression significantly increased the expression of p-Akt, Akt, Bcl-2, Wnt, ß-catenin, matrix metalloprotein-9 (MMP-9), and epidermal growth factor (EGFR) levels, and decreased Bax expression. The regulatory role of miR-21 inhibitor-EGFP was opposite to that of miR-21-EGFP. After UTMD, miR-21-EGFP and miR-21 inhibitor-EGFP had more significant regulatory effects on these genes. CONCLUSIONS Our research revealed that an ultrasound intensity of 1.5 W/cm² is the best parameter for miR-21 transfection. UTMD can enhance the biological function of miR-21 in HeLa cells, and alter the effect of miR-21 on apoptosis, metastasis, and phosphorylation genes.
Conflict of interest statement
None.
Figures

Similar articles
-
Ultrasound-targeted microbubble destruction mediated miR-492 inhibitor suppresses the tumorigenesis in non-small cell lung cancer.Ann Med. 2021 Dec;53(1):2246-2255. doi: 10.1080/07853890.2021.2005254. Ann Med. 2021. PMID: 34818961 Free PMC article.
-
Ultrasound-Targeted Microbubble Destruction-Mediated miR-206 Overexpression Promotes Apoptosis and Inhibits Metastasis of Hepatocellular Carcinoma Cells Via Targeting PPIB.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820959355. doi: 10.1177/1533033820959355. Technol Cancer Res Treat. 2020. PMID: 33111654 Free PMC article.
-
Ultrasound-Targeted Microbubble Destruction-Mediated miR-1228 Downregulation Suppresses Tumor Proliferation, Migration, and Invasion of Cervical-Cancer Cells.Gynecol Obstet Invest. 2022;87(3-4):211-218. doi: 10.1159/000525594. Epub 2022 Jun 21. Gynecol Obstet Invest. 2022. PMID: 35728571
-
Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles.Cardiovasc Ultrasound. 2013 Apr 17;11:11. doi: 10.1186/1476-7120-11-11. Cardiovasc Ultrasound. 2013. PMID: 23594865 Free PMC article. Review.
-
New progress in angiogenesis therapy of cardiovascular disease by ultrasound targeted microbubble destruction.Biomed Res Int. 2014;2014:872984. doi: 10.1155/2014/872984. Epub 2014 May 12. Biomed Res Int. 2014. PMID: 24900995 Free PMC article. Review.
Cited by
-
Effect of Gambogic Acid-Loaded Porous-Lipid/PLGA Microbubbles in Combination With Ultrasound-Triggered Microbubble Destruction on Human Glioma.Front Bioeng Biotechnol. 2021 Sep 15;9:711787. doi: 10.3389/fbioe.2021.711787. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34604184 Free PMC article.
References
-
- Cadron I, Van Gorp T, Amant F, et al. Chemotherapy for recurrent cervical cancer. Gynecol Oncol. 2007;107(1 Suppl 1):S113–18. - PubMed
-
- Angioli R, Plotti F, Montera R, et al. Neoadjuvant chemotherapy plus radical surgery followed by chemotherapy in locally advanced cervical cancer. Gynecol Oncol. 2012;127(2):290–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

