Germline molecular data in hereditary breast cancer in Brazil: Lessons from a large single-center analysis
- PMID: 33606809
- PMCID: PMC7895369
- DOI: 10.1371/journal.pone.0247363
Germline molecular data in hereditary breast cancer in Brazil: Lessons from a large single-center analysis
Abstract
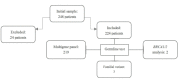
Brazil is the largest country in South America and the most genetically heterogeneous. The aim of the present study was to determine the prevalence of germline pathogenic variants (PVs) in Brazilian patients with breast cancer (BC) who underwent genetic counseling and genetic testing at a tertiary Oncology Center. We performed a retrospective analysis of the medical records of Brazilian patients with BC referred to genetic counseling and genetic testing between August 2017 and August 2019. A total of 224 unrelated patients were included in this study. Premenopausal women represented 68.7% of the cohort. The median age at BC diagnosis was 45 years. Multigene panel testing was performed in 219 patients, five patients performed single gene analysis or family variant testing. Forty-eight germline PVs distributed among 13 genes were detected in 20.5% of the patients (46/224). Eighty-five percent of the patients (91/224) fulfilled NCCN hereditary BC testing criteria. Among these patients, 23.5% harbored PVs (45/191). In the group of patients that did not meet NCCN criteria, PV detection rate was 3% (1/33). A total of 61% of the patients (28/46) harbored a PV in a high-penetrance BC gene: 19 (8.5%) BRCA1/2, 8 (3.5%) TP53, 1 (0.5%) PALB2. Moderate penetrance genes (ATM, CHEK2) represented 15.2% (7/46) of the positive results. PVs detection was statistically associated (p<0.05) with BC diagnosis before age 45, high-grade tumors, bilateral BC, history of multiple primary cancers, and family history of pancreatic cancer. According to the current hereditary cancer guidelines, 17.4% (39/224) of the patients had actionable variants. Nine percent of the patients (20/224) had actionable variants in non-BRCA genes, it represented 43.5% of the positive results and 51.2% of the actionable variants. Considering the observed prevalence of PVs in actionable genes beyond BRCA1/2 (9%, 20/224), multigene panel testing may offer an effective first-tier diagnostic approach in this population.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Hoang LN, Gilks BC. Hereditary Breast and Ovarian Cancer Syndrome: Moving Beyond BRCA1 and BRCA2. Adv Anat Pathol [Internet]. 2018. March;25(2):85–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20301425%5Cnhttp://www.ncbi.nlm.nih.g... 10.1097/PAP.0000000000000177 - DOI - PubMed
-
- Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020;38(18):2080–106. 10.1200/JCO.20.00299 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

