No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial
- PMID: 33607104
- PMCID: PMC7885705
- DOI: 10.1016/j.ejphar.2021.173947
No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial
Abstract
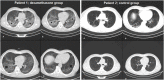
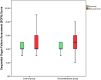
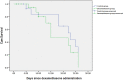
The aim of this study was to evaluate the clinical effects of dexamethasone administration in patients with mild to moderate acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19). The study included 50 patients who were randomly assigned to the dexamethasone group or control group. Dexamethasone was administered at a dose of 20 mg/day from day 1-5 and then at 10 mg/day from day 6-10. The need for invasive mechanical ventilation, death rate, duration of clinical improvement, length of hospital stay, and radiological changes in the computed tomography scan were assessed. The results revealed that 92% and 96% of patients in the dexamethasone and control groups, respectively, required noninvasive ventilation (P = 0.500). Among them, 52% and 44% of patients in the dexamethasone and control groups, respectively, required invasive mechanical ventilation (P = 0.389). At the end of the study, 64% of patients in the dexamethasone group and 60% of patients in the control group died (P = 0.500); the remaining patients were discharged from the hospital during the 28-day follow-up period. The median length of hospital stay was 11 days in the dexamethasone group and 6 days in the control group (P = 0.036) and the median length of hospital stay was 7 days in the dexamethasone group and 3 days in the control group (P < 0.001). No significant differences were observed in the other outcomes. This study showed that corticosteroid administration had no clinical benefit in patients with COVID-19-induced mild to moderate ARDS.
Keywords: COVID-19; Corticosteroids; Dexamethasone; SARS-Cov-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures
References
-
- Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.-P., Chu H., Zhou J., Chen H., Qin C., Yuen K.-Y. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212:1904–1913. - PMC - PubMed
-
- Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.22.20040758. - DOI
-
- DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., Castaño-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-κB-Mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

