Comparative analysis of point-of-care, high-throughput and laboratory-developed SARS-CoV-2 nucleic acid amplification tests (NATs)
- PMID: 33607117
- PMCID: PMC7885623
- DOI: 10.1016/j.jviromet.2021.114102
Comparative analysis of point-of-care, high-throughput and laboratory-developed SARS-CoV-2 nucleic acid amplification tests (NATs)
Abstract
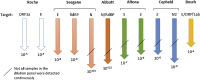
Multiple nucleic acid amplification tests (NATs) are available for the detection of SARS-CoV-2 in clinical specimens, including Laboratory Developed Tests (LDT), commercial high-throughput assays and point-of-care tests. Some assays were just recently released and there is limited data on their clinical performance. We compared the Xpert® Xpress SARS-CoV-2 (Cepheid) and Vivalytic VRI Panel (Schnelltest COVID-19) (Bosch) point-of-care tests with four high-throughput assays and one LDT, the cobas® SARS-CoV-2 test (Roche), the Allplex™ 2019-nCoV Assay (Seegene), the SARS-CoV-2 AMP (Abbott) Kit, the RealStar® SARS-CoV-2 RT-PCR Kit 1.0 (altona) as well as an assay using a SARS-CoV-2 RdRP gene specific primer and probe set. Samples from patients with confirmed SARS-CoV-2 infection, samples from the first and second SARS-CoV-2-PCR External Quality Assessment (EQA) (INSTAND e.V.) and a 10-fold serial dilution of a SARS-CoV-2 cell culture (SARS-CoV-2 Frankfurt 1) supernatant were examined. We determined that the NAT assays examined had a high specificity. Assays using the N gene as target demonstrated the highest sensitivity in the serial dilution panel, while all examined NAT assays showed a comparable sensitivity when testing clinical and EQA samples.
Keywords: NAT; PCR; POCT; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors have competing interests related to this work.
Figures
References
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) - PMC - PubMed
-
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., Adriano A., Beese S., Dretzke J., Di Ferrante Ruffano L., Harris I.M., Price M.J., Dittrich S., Emperador D., Hooft L., Leeflang M.M., van den Bruel A. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6 - PMC - PubMed
-
- Kohmer N., Toptan T., Pallas C., Karaca O., Pfeiffer A., Westhaus S., Widera M., Berger A., Hoehl S., Kammel M., Ciesek S., Rabenau H.F. The Comparative Clinical Performance of Four SARS-CoV-2 Rapid Antigen Tests and Their Correlation to Infectivity In Vitro. J. Clin. Med. 2021;10(2):328. doi: 10.3390/jcm10020328. - DOI - PMC - PubMed
-
- La Scola B., Le Bideau M., Andreani J., van Hoang T., Grimaldier C., Colson P., Gautret P., Raoult D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1059–1061. - PMC - PubMed
-
- Lambert-Niclot S., Cuffel A., Le Pape S., Vauloup-Fellous C., Morand-Joubert L., Roque-Afonso A.-M., Le Goff J., Delaugerre C. Evaluation of a rapid diagnostic assay for detection of SARS CoV-2 antigen in nasopharyngeal swab. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.00977-20. e00977-20. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

