Outcomes After Use of a Lymph Node Collection Kit for Lung Cancer Surgery: A Pragmatic, Population-Based, Multi-Institutional, Staggered Implementation Study
- PMID: 33607311
- PMCID: PMC8012255
- DOI: 10.1016/j.jtho.2020.12.025
Outcomes After Use of a Lymph Node Collection Kit for Lung Cancer Surgery: A Pragmatic, Population-Based, Multi-Institutional, Staggered Implementation Study
Abstract
Introduction: Suboptimal pathologic nodal staging prevails after curative-intent resection of lung cancer. We evaluated the impact of a lymph node specimen collection kit on lung cancer surgery outcomes in a prospective, population-based, staggered implementation study.
Methods: From January 1, 2014, to August 28, 2018, we implemented the kit in three homogeneous institutional cohorts involving 11 eligible hospitals from four contiguous hospital referral regions. Our primary outcome was pathologic nodal staging quality, defined by the following evidence-based measures: the number of lymph nodes or stations examined, proportions with poor-quality markers such as nonexamination of lymph nodes, and aggregate quality benchmarks including the National Comprehensive Cancer Network criteria. Additional outcomes included perioperative complications, health care utilization, and overall survival.
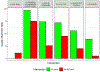
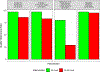
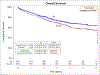
Results: Of 1492 participants, 56% had resection with the kit and 44% without. Pathologic nodal staging quality was significantly higher in the kit cases: 0.2% of kit cases versus 9.8% of nonkit cases had no lymph nodes examined; 3.2% versus 25.3% had no mediastinal lymph nodes; 75% versus 26% attained the National Comprehensive Cancer Network criteria (p < 0.0001 for all comparisons). Kit cases revealed no difference in perioperative complications or health care utilization except for significantly shorter duration of surgery, lower proportions with atelectasis, and slightly higher use of blood transfusion. Resection with the kit was associated with a lower hazard of death (crude, 0.78 [95% confidence interval: 0.61-0.99]; adjusted 0.85 [0.71-1.02]).
Conclusions: Lung cancer surgery with a lymph node collection kit significantly improved pathologic nodal staging quality, with a trend toward survival improvement, without excessive perioperative morbidity or mortality.
Keywords: Lymph node specimen collection kit; Lymphadenectomy; Nodal staging; Quality of surgical care; Surgical resection.
Copyright © 2021 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Comparative Effectiveness of a Lymph Node Collection Kit Versus Heightened Awareness on Lung Cancer Surgery Quality and Outcomes.J Thorac Oncol. 2021 May;16(5):774-783. doi: 10.1016/j.jtho.2021.01.1618. Epub 2021 Feb 12. J Thorac Oncol. 2021. PMID: 33588112 Free PMC article.
-
Institution-Level Evolution of Lung Cancer Resection Quality With Implementation of a Lymph Node Specimen Collection Kit.J Thorac Oncol. 2023 Jul;18(7):858-868. doi: 10.1016/j.jtho.2023.03.002. Epub 2023 Mar 15. J Thorac Oncol. 2023. PMID: 36931504 Free PMC article.
-
Effectiveness of Implemented Interventions on Pathologic Nodal Staging of Non-Small Cell Lung Cancer.Ann Thorac Surg. 2018 Jul;106(1):228-234. doi: 10.1016/j.athoracsur.2018.02.021. Epub 2018 Mar 11. Ann Thorac Surg. 2018. PMID: 29534956 Free PMC article.
-
ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer.Eur J Cardiothorac Surg. 2006 Nov;30(5):787-92. doi: 10.1016/j.ejcts.2006.08.008. Epub 2006 Sep 12. Eur J Cardiothorac Surg. 2006. PMID: 16971134
-
Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer.Ann Thorac Surg. 2007 Sep;84(3):1059-65. doi: 10.1016/j.athoracsur.2007.04.032. Ann Thorac Surg. 2007. PMID: 17720443 Review.
Cited by
-
Advancing rapid cycle research in cancer care delivery: a National Cancer Institute workshop report.J Natl Cancer Inst. 2023 May 8;115(5):498-504. doi: 10.1093/jnci/djad007. J Natl Cancer Inst. 2023. PMID: 36637203 Free PMC article.
-
Surgeon Quality and Patient Survival After Resection for Non-Small-Cell Lung Cancer.J Clin Oncol. 2023 Jul 10;41(20):3616-3628. doi: 10.1200/JCO.22.01971. Epub 2023 Jun 2. J Clin Oncol. 2023. PMID: 37267506 Free PMC article.
-
Identification of Factors Related to the Quality of Lymphadenectomy for Lung Cancer: Secondary Analysis of Prospective Randomized Trial Data.J Clin Med. 2023 May 31;12(11):3780. doi: 10.3390/jcm12113780. J Clin Med. 2023. PMID: 37297976 Free PMC article.
-
Influencing Factors on the Quality of Lymph Node Dissection for Stage IA Non-Small Cell Lung Cancer: A Retrospective Nationwide Cohort Study.Cancers (Basel). 2024 Jan 13;16(2):346. doi: 10.3390/cancers16020346. Cancers (Basel). 2024. PMID: 38254835 Free PMC article.
-
Two Interventions on Pathologic Nodal Staging in a Population-Based Lung Cancer Resection Cohort.Ann Thorac Surg. 2024 Mar;117(3):576-584. doi: 10.1016/j.athoracsur.2023.08.026. Epub 2023 Sep 5. Ann Thorac Surg. 2024. PMID: 37678613 Free PMC article.
References
-
- Pfannschmidt J, Muley T, Bulzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 55:371–377, 2007 - PubMed
-
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol 10:1675–1684, 2015 - PubMed
-
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 80:2051–2056, 2005 - PubMed
-
- Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg 96:1178–1189, 2013 - PubMed
-
- Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 7:1798–1806, 2012 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

