Ten Years of Newborn Screening for Severe Combined Immunodeficiency (SCID) in Massachusetts
- PMID: 33607339
- PMCID: PMC8113135
- DOI: 10.1016/j.jaip.2021.02.006
Ten Years of Newborn Screening for Severe Combined Immunodeficiency (SCID) in Massachusetts
Abstract
Background: Massachusetts began newborn screening (NBS) for severe combined immunodeficiency (SCID) using measurement of T-cell receptor excision circles (TRECs) from dried blood spots.
Objective: We describe developments and outcomes from the first 10 years of this program (February 1, 2009, to January 31, 2019).
Methods: TREC values, diagnostic, and outcome data from all patients screened for SCID were evaluated.
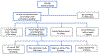
Results: NBS of 720,038 infants prompted immunologic evaluation of 237 (0.03%). Of 237, 9 were diagnosed with SCID/leaky SCID (4% of referrals vs 0.001% general population). Another 7 were diagnosed with other combined immunodeficiencies, and 3 with athymia. SCID/leaky SCID incidence was approximately 1 in 80,000, whereas approximately 1 in 51,000 had severe T-cell lymphopenia for which definitive treatment was indicated. All patients with SCID/leaky SCID underwent hematopoietic cell transplant or gene therapy with 100% survival. One patient with athymia underwent successful thymus transplant. No known cases of SCID were missed. Compared with outcomes from the 10 years before SCID NBS, survival trended higher (9 of 9 vs 4 of 7), likely due to a lower rate of infection before treatment.
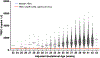
Conclusions: Our data support a single NBS testing-and-referral algorithm for all gestational ages. Despite lower median TREC values in premature infants, the majority for all ages are well above the TREC cutoff and the algorithm, which selects urgent (undetectable TREC) and repeatedly abnormal TREC values, minimizes referral. We also found that low naïve T-cell percentage is associated with a higher risk of SCID/CID, demonstrating the utility of memory/naïve T-cell phenotyping as part of follow-up flow cytometry.
Keywords: Athymia; Hematopoietic cell transplant (HCT); Newborn screening (NBS); Prematurity; SCID; TREC.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: All authors attest that they have no conflicts to disclose.
Figures


References
-
- Association of Public Health Laboratories. Severe Combined Immunodeficiency 2020. [Available from: https://www.newsteps.org/disorders/scid.
-
- Center for Disease Control and Prevention. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Morb Mortal Wkly Rep. 2004;53:1–29. - PubMed
-
- Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

